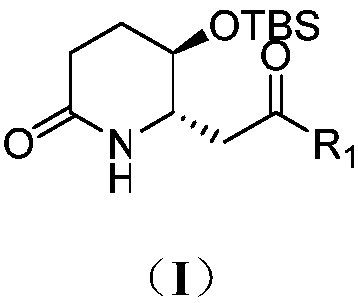
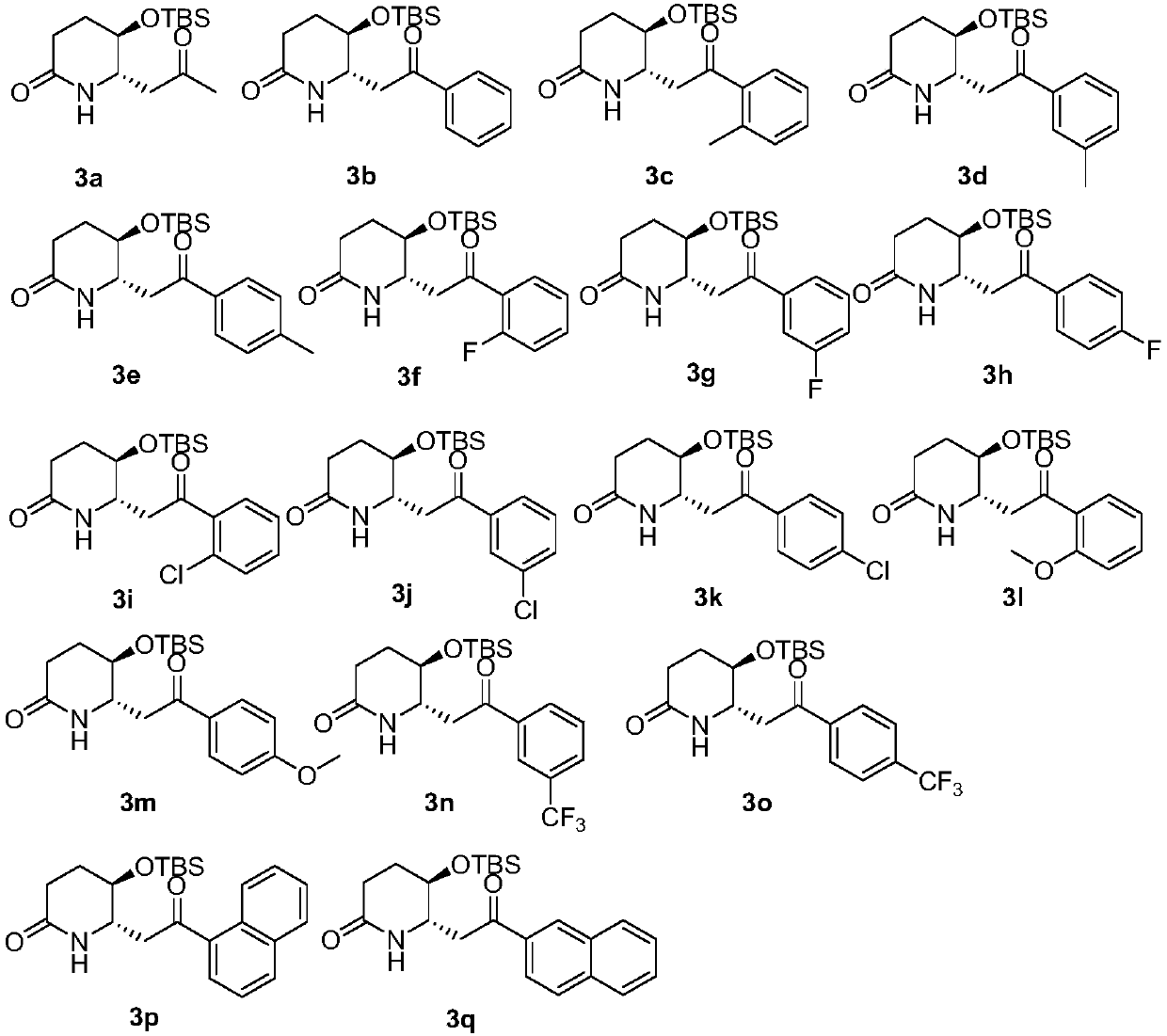
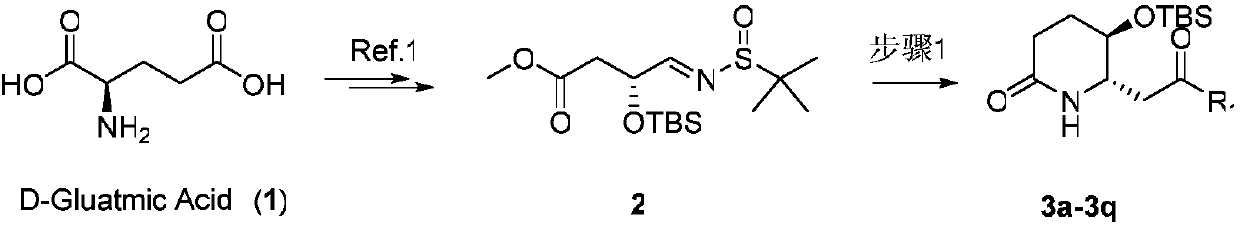
3-hydroxy-2-piperidineamide framework febrifugine (halofuginone) and preparation method thereof
A technology of piperidinamide and tembrolizine, which is applied in the fields of 3-hydroxy-2-piperidine amide skeleton kefir, 3-hydroxy 2-piperidine amide skeleton kefir compound and analogs thereof and the fields of preparation thereof, and can solve the complicated route , difficult to operate, expensive and other problems, to achieve the effect of simple reaction conditions, simple route and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of compound 3a
[0039] (5R,6S)-5-((tert-Butyldimethylsilyl)oxy)-6-(2-oxopropyl)piperidin-2-one(3a)
[0040] Lithium diisopropylamide (8.3mmol, 10eq) was newly prepared under nitrogen protection at room temperature, diluted with anhydrous tetrahydrofuran solution (5mL), injected into acetone reagent (8.3mmol, 10eq) and reacted at room temperature for 0.5 hours. The newly generated negative ion was reacted with compound 2 (300mg, 0.83mmol, 1.0eq) at a low temperature of -78°C for 2.5 hours, then quenched by adding saturated ammonium chloride, extracted, concentrated, and then added 5mL of hydrochloric acid / dioxane solution in After reacting at room temperature for 0.5 hours to detect the completion of the reaction, spin dry on a rotary evaporator. After spin-drying, add sodium hydroxide solution (4M, 5mL) directly to it without purification, react at room temperature for 5 minutes, extract with ethyl acetate, wash with saturated brine, dry with anhydrous magne...
Embodiment 2
[0090] Synthesis of compound 3a
[0091] (5R,6S)-5-((tert-Butyldimethylsilyl)oxy)-6-(2-oxopropyl)piperidin-2-one(3a)
[0092] Lithium diisopropylamide (8.3mmol, 10eq) was newly prepared under nitrogen protection at room temperature, diluted with anhydrous tetrahydrofuran solution (5mL), injected into acetone reagent (8.3mmol, 10eq) and reacted at room temperature for 0.5 hours. The newly generated negative ion was reacted with compound 2 (300mg, 0.83mmol, 1.0eq) at a low temperature of -78°C for 2.5 hours, then quenched by adding saturated ammonium chloride, extracted, concentrated, added anhydrous methanol (20mL) and passed through grass Acyl chloride (16.6mmol, 20eq) was quenched, and then reacted at room temperature for 0.5 hours to check that the reaction was complete, and then spin-dried on a rotary evaporator. After spin-drying, add sodium hydroxide solution (4M, 50mL) directly to it without purification, react at room temperature for 5 minutes, extract with ethyl aceta...
Embodiment 3
[0094] Synthesis of compound 3a
[0095] (5R,6S)-5-((tert-Butyldimethylsilyl)oxy)-6-(2-oxopropyl)piperidin-2-one(3a)
[0096]Lithium diisopropylamide (1.25mmol, 1.5eq) was newly prepared under nitrogen protection at room temperature, diluted with anhydrous tetrahydrofuran solution (2mL), injected with acetone reagent (0.83mmol, 1.0eq) and reacted at room temperature for 1 hour. The newly generated negative ion was reacted with compound 2 (300mg, 0.83mmol, 1.0eq) at a low temperature of -78°C for 2.5 hours, then anhydrous methanol (5mL) was added and quenched with oxalyl chloride (1.66mmol, 2.0eq), and then After reacting at room temperature for 0.5 hours to detect the completion of the reaction, spin dry on a rotary evaporator. After spin-drying, add sodium hydroxide solution (4M, 5mL) directly to it without purification, react at room temperature for 5 minutes, extract with ethyl acetate, wash with saturated brine, dry with anhydrous magnesium sulfate, filter with suction, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com