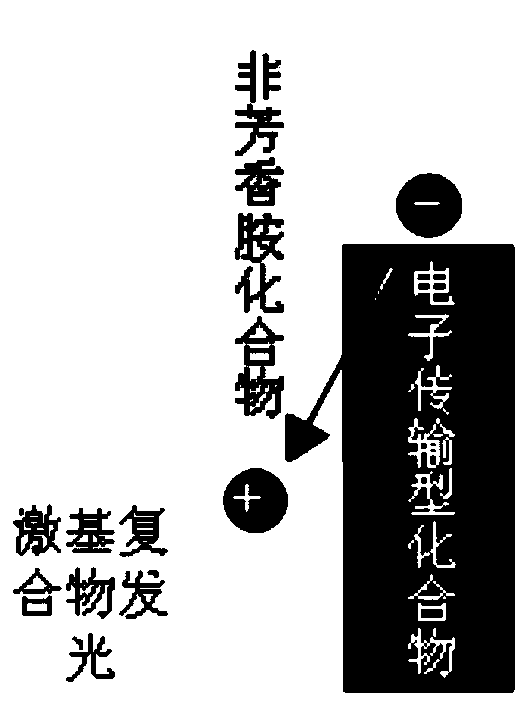
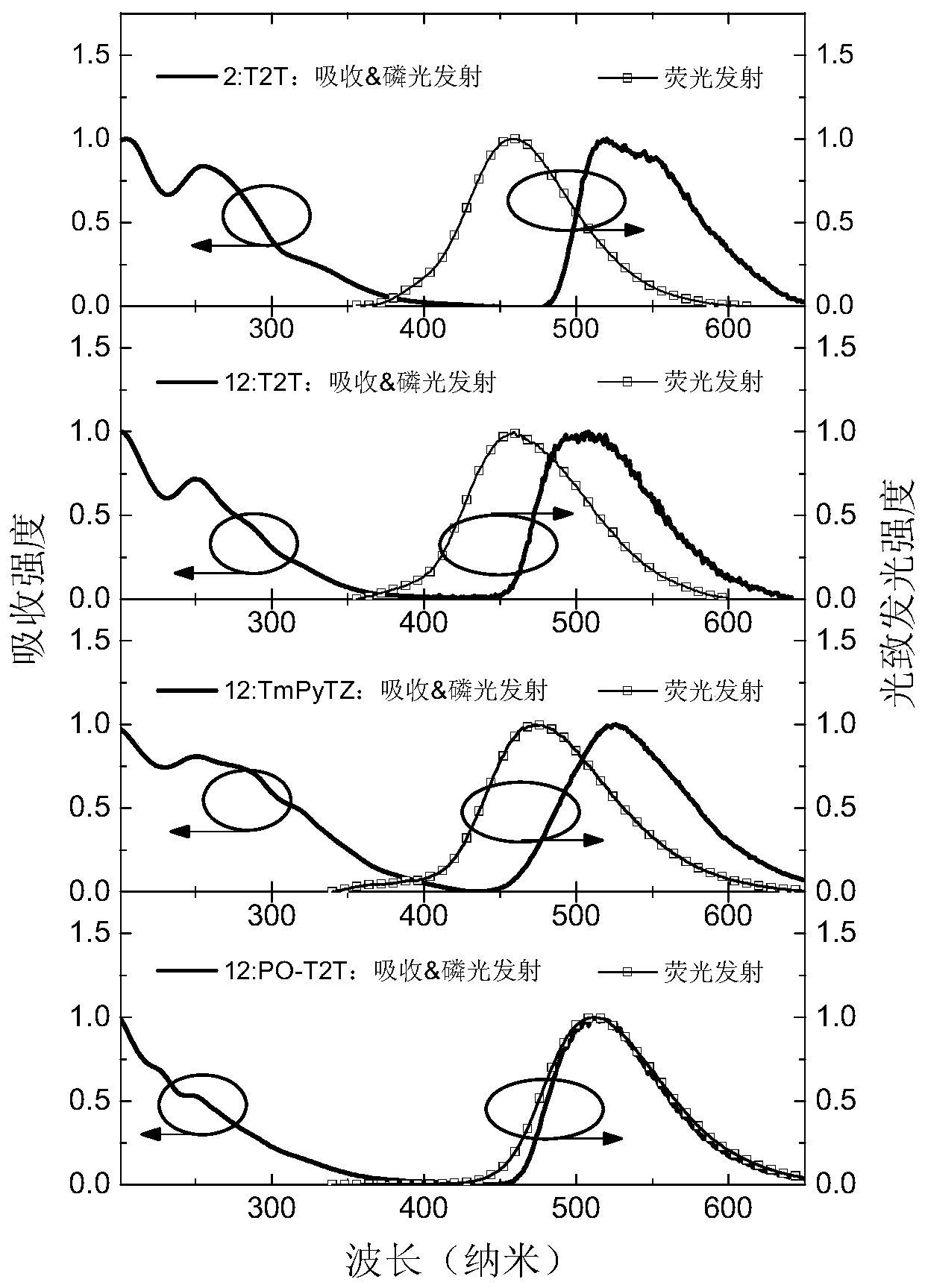
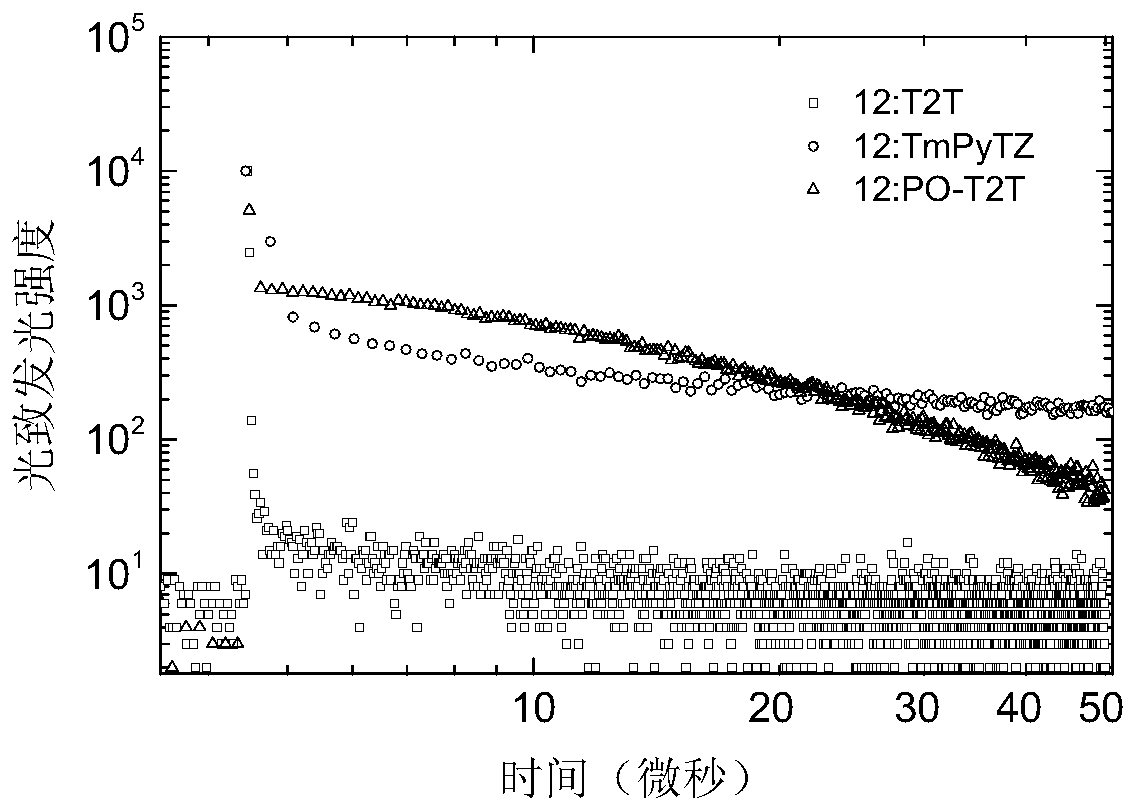
Non-aromatic amine small molecule photoelectric material and preparation and application thereof
A technology of aromatic amines and optoelectronic materials, which is applied in the direction of luminescent materials, circuits, electrical components, etc., can solve the problems that the exciplex system has not been reported, and achieve the effect of good carrier transfer characteristics and high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The reaction formula of intermediate 1 is as follows:
[0037]
[0038] Concrete reaction steps are as follows:
[0039] Thianthrene (5mmol, 1.08g) was added to a reaction flask containing 100mL of acetic acid, and liquid bromine (5mmol, 800mg) was slowly added dropwise. After passing nitrogen gas for 15 minutes, the reaction was heated at 80° C. for 5 hours. After the reaction, when the system returned to room temperature, sodium bisulfite was added to quench the reaction, extracted with dichloromethane and saturated brine, the organic phase was recovered, and the solvent was distilled off under reduced pressure. The crude product was separated and purified by column chromatography, the eluent was petroleum ether / dichloromethane 5:1, and the obtained intermediate product was dried overnight and dissolved in 60 mL of tetrahydrofuran in the reactor, followed by adding 2-isopropoxy- 4,4,5,5-tetramethyl-1,3,2-dioxaborolane (5mmol, 0.82mL), slowly drop n-butyllithium (...
Embodiment 2
[0041] The reaction formula of intermediate 2 is as follows:
[0042]
[0043] Concrete reaction steps are as follows:
[0044]Compared with intermediate 1, the difference is that thianthrene is replaced by an equivalent amount of dibenzo-p-dioxin, and other raw materials and steps are the same as intermediate 1. Finally, intermediate 2 was obtained with a yield of 92%. Intermediate 2 Molecular formula: C 18 h 19 BO 4 ; Molecular weight: m / z: 310.14; Elemental analysis results: C, 69.71; H, 6.17; B, 3.49; O, 20.63.
Embodiment 3
[0046] The reaction formula of intermediate 3 is as follows:
[0047]
[0048] Concrete reaction steps are as follows:
[0049] Add 2-cyanothiophenol (10mmol, 1.26g), 1,4-dibromo-dinitrobenzene (10mmol, 2.78g), potassium tert-butoxide (30mmol, 3.5g), and DMF 100mL into the reactor in sequence In the process, after passing nitrogen gas for 30 minutes, the reaction was carried out under heating at 160° C. for 24 hours. After the reaction, the system was returned to room temperature, extracted with dichloromethane and saturated brine, the organic phase was recovered, and the solvent was distilled off under reduced pressure. The crude product was separated and purified by column chromatography. The eluent was petroleum ether / dichloromethane 5:1. The obtained intermediate product was dried overnight and dissolved in 80 mL of tetrahydrofuran in the reactor, and then 2-isopropoxy- 4,4,5,5-tetramethyl-1,3,2-dioxaborolane (10mmol, 1.64mL), slowly drop n-butyl lithium (7.5mmol, 5mL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com