Novel MCO (multicopper oxidase) with function of efficient degradation of histamine
A multi-copper oxidase and biogenic amine technology, applied in the field of molecular biology, can solve the problems of neutral optimum pH, few types of biogenic amines for degradation, and low efficiency of histamine, and achieve the effect of improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] According to the gene sequence of multi-copper oxidase in Lactobacillus fermentum strain 47-7genome (GenBank accession number: NZ_CP017712.1) in NCBI, primers were designed, and the genome of Lactobacillus fermentum (Lactobacillus fermentum) preserved in our laboratory was used as a template to amplify Increased copper oxidase gene. The primers required for amplification are as follows:
[0038] Upstream: ATGAATGAACCAGTTTTTCGATTC
[0039] Downstream: CTACATGTGCATCCCCATCTT
[0040] PCR reaction program: pre-denaturation at 95°C for 3 min; denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min and 30 s, 34 cycles; extension at 72°C for 10 min, and incubation at 4°C.
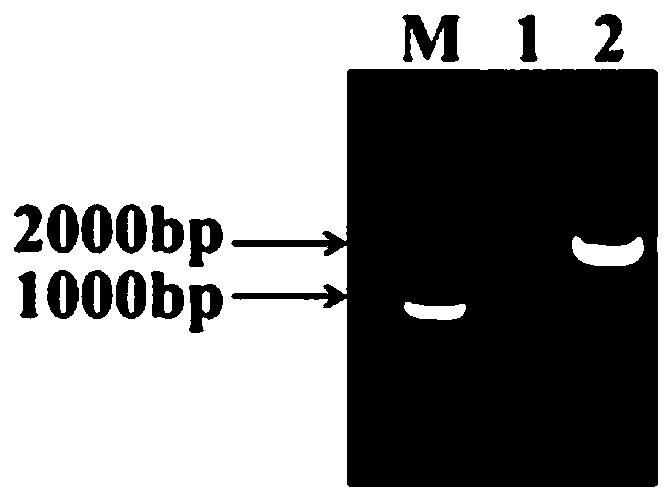
[0041] After the amplification is completed, it is verified by 1% agarose gel electrophoresis, such as figure 1 As shown, the amplified gene fragment with a size of about 1500bp is consistent with the size of the target gene, indicating that L. fermentum contains a multi-co...
Embodiment 2
[0043] (1) Vector construction: the gene obtained in Example 1 and pET-28a were double-digested with restriction endonucleases EcoRI and HindIII, and reacted in a metal bath at 37°C for 45 minutes. After the digested gene fragments and plasmids were recovered and purified, they were mixed at a molar ratio of 4-10:1, ligated by Solution I ligase, and ligated overnight in a metal bath at 16°C.
[0044] (2) Construction of recombinant bacteria: 5 μL of the ligation product prepared in step (1) was added to Escherichia coli JM109 competent cells, mixed evenly, and left to stand in ice for 30 minutes. After 90 seconds in a water bath at 42°C, immediately take it out and place it in ice for 2-5 minutes. Add 700μL LB to culture based on 37℃, 200r min -1 Shake culture for 1h. 4000r·min -1 Centrifuge for 2 minutes, discard most of the supernatant, and leave about 100 μL of the supernatant to resuspend the bacteria. The bacterial solution was evenly spread on a plate containing kana...
Embodiment 3
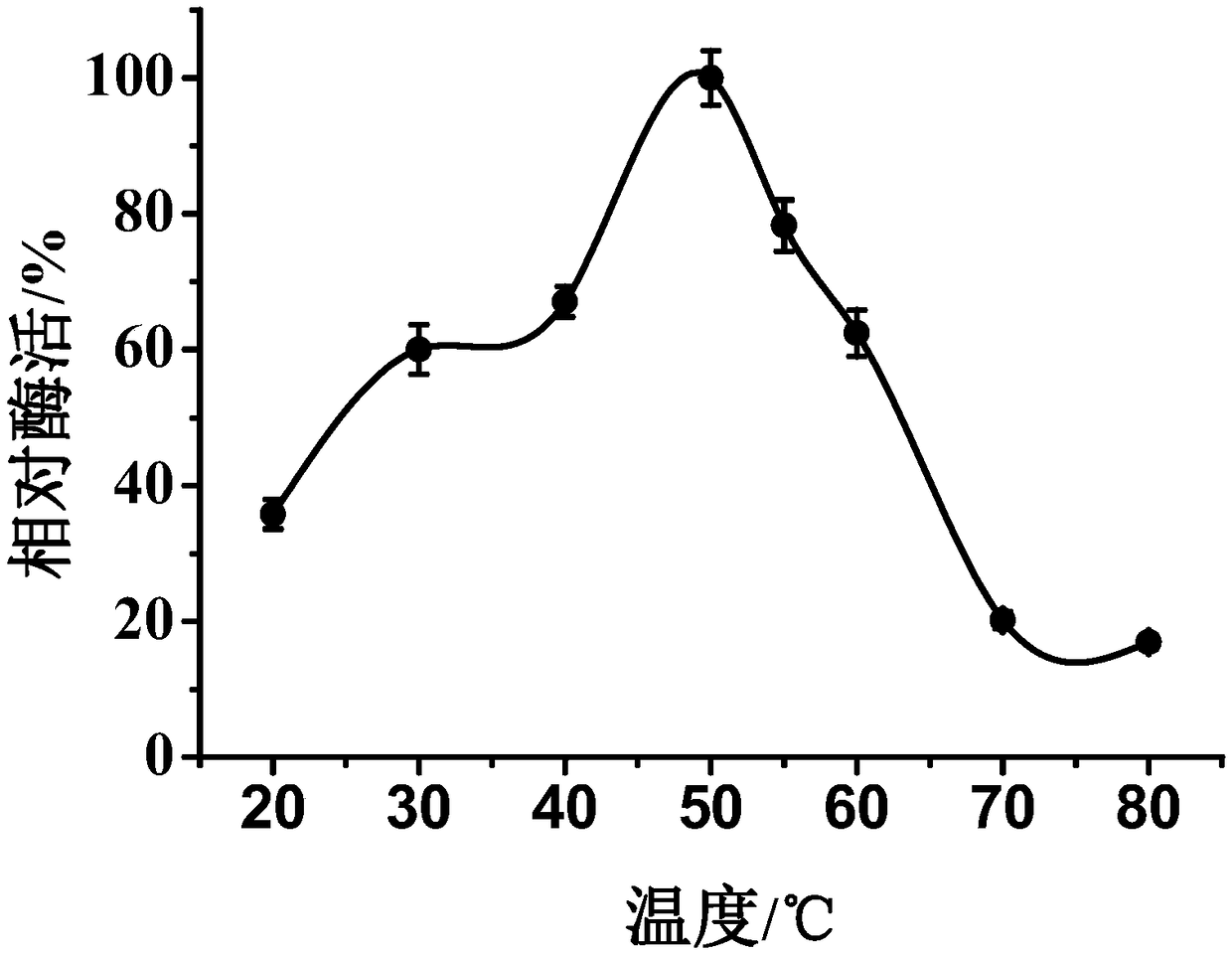
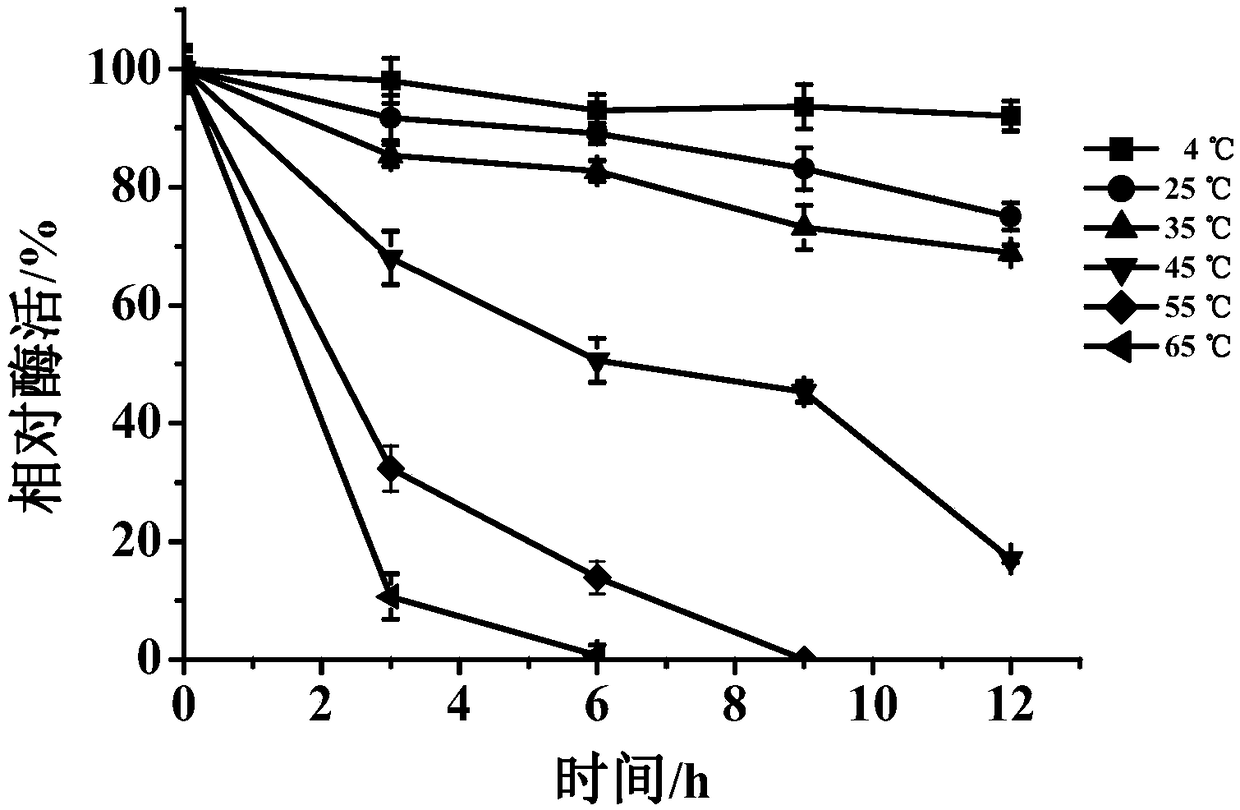
[0050] (1) Effect of temperature on enzyme activity and stability
[0051] The purified multi-copper oxidase was reacted with the substrate under different temperature conditions (25, 35, 40, 45, 50, 55, 60, 70, 80°C) to measure the enzyme activity, and the highest enzyme activity was taken as 100 %, calculate the relative enzyme activity at each temperature to determine the optimal reaction temperature of the enzyme.
[0052] The purified enzyme solution was warmed under various temperature conditions, and an appropriate amount of enzyme solution was taken every 3 hours to react with the substrate at the optimum reaction temperature, and the influence of different temperature conditions on the stability of the enzyme activity was determined.
[0053] Such as figure 2 As shown, when the temperature of MCOF is 30-60°C, the enzyme activity can reach more than 50%, and when the temperature is 50°C, the relative enzyme activity can reach 100%; image 3 As shown, when MCOF is lo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com