Application of intestinal microorganisms as diagnostic markers of cholangiocarcinoma
A technology of intestinal microbes and cholangiocarcinoma, which is applied in the field of microbial markers of cholangiocarcinoma, and can solve the problems that there are no studies on intestinal flora biomarkers of cholangiocarcinoma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
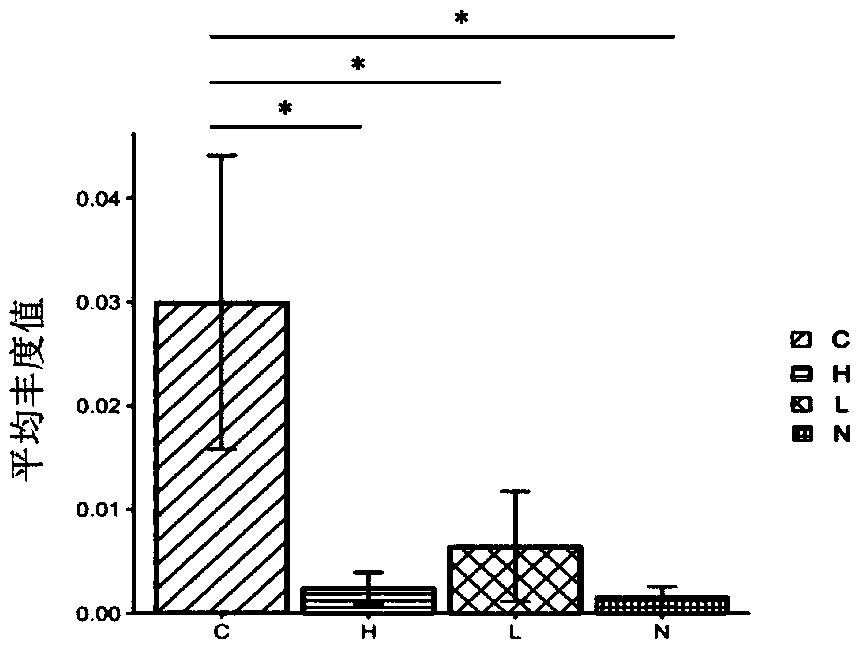
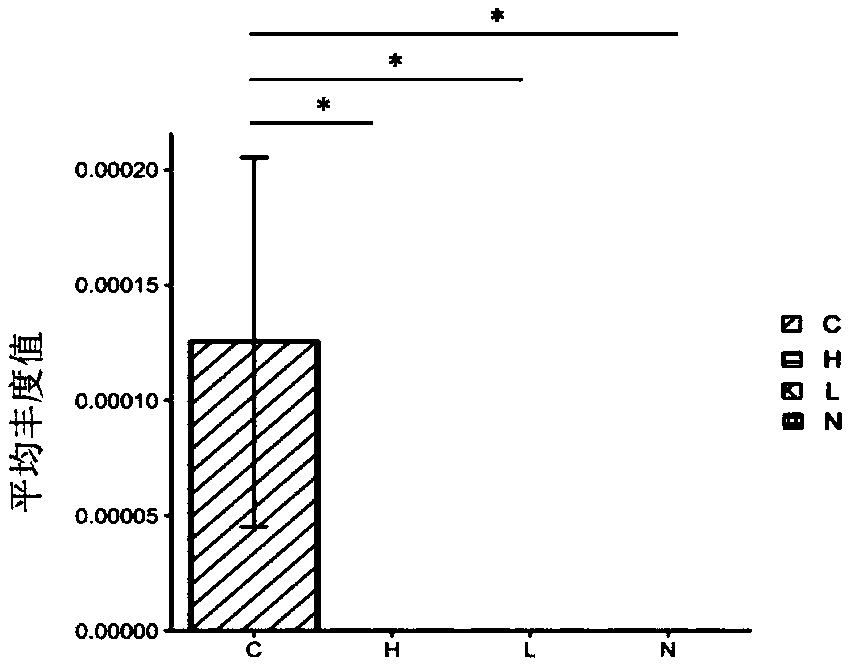
[0059] Example 1 Detection of differences in the abundance of intestinal microbial flora in cholangiocarcinoma, hepatocellular carcinoma, liver cirrhosis, and healthy people
[0060] 1. Research object
[0061] Patients with cholangiocarcinoma, hepatocellular carcinoma and liver cirrhosis were collected and confirmed by liver biopsy.
[0062] The patient information and healthy person information are shown in Table 1.
[0063] Table 1 Clinical information
[0064]
[0065]
[0066] 2. Feces collection and treatment
[0067] Fresh, middle and late stool samples were collected and immediately frozen in a -80°C freezer.
[0068] 3. Nucleic acid extraction
[0069] Stool sample DNA according to MOBIO DNA Isolation Kit 12888-100 instruction manual for extraction. The general steps are as follows:
[0070] (1) Add 0.25g of feces sample to a powerBead Tubes, vortex gently;
[0071] (2) Add 60μl Solution C1, mix up and down several times;
[0072] (3) Fix the powerBead...
Embodiment 2
[0110] Example 2 Clinical diagnostic value of Lactobacillus and Alloscardovia
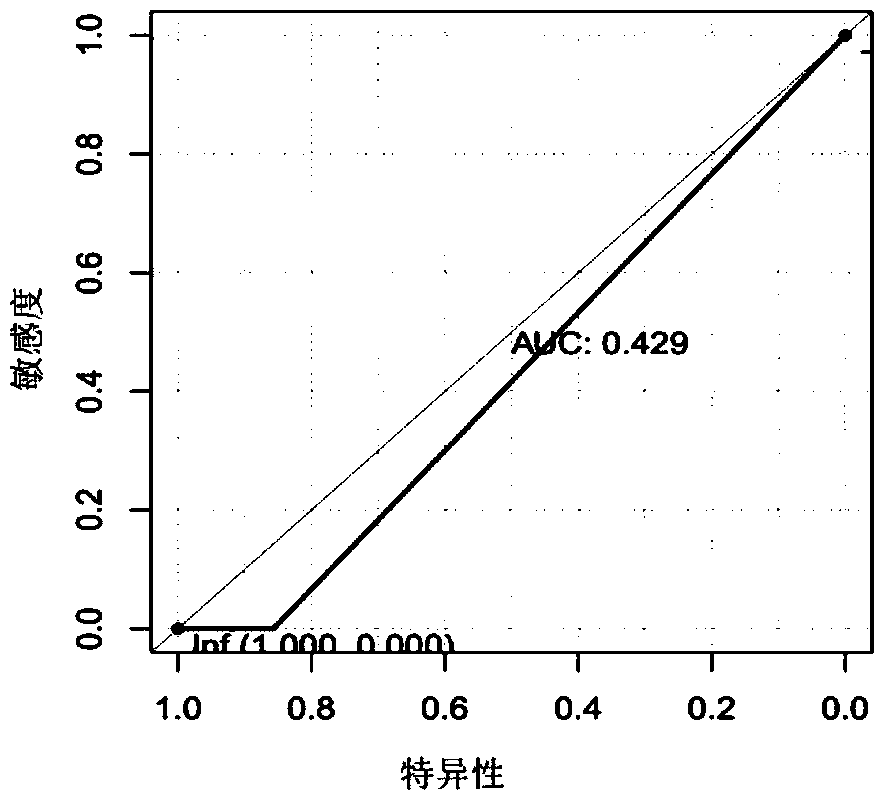
[0111] According to the abundance data obtained in Example 1, a ROC curve was made to analyze the clinical diagnostic value of Lactobacillus and Alloscardovia.
[0112] result:
[0113] image 3 The results showed that when Lactobacillus genus differentiated cholangiocarcinoma and hepatocellular carcinoma, the AUC was 0.429;
[0114] Figure 4 When showing Lactobacillus to distinguish cholangiocarcinoma from liver cirrhosis, the AUC was 0.729;
[0115] Figure 5 When showing Lactobacillus to distinguish cholangiocarcinoma from normal people, the AUC was 0.679;
[0116] Figure 6 When showing Alloscardovia genus to distinguish cholangiocarcinoma from hepatocellular carcinoma, the AUC was 0.798;
[0117] Figure 7 When showing Alloscardovia genus to distinguish cholangiocarcinoma from liver cirrhosis, the AUC was 0.729;
[0118] Figure 8 When showing Alloscardovia to distinguish cholangioc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com