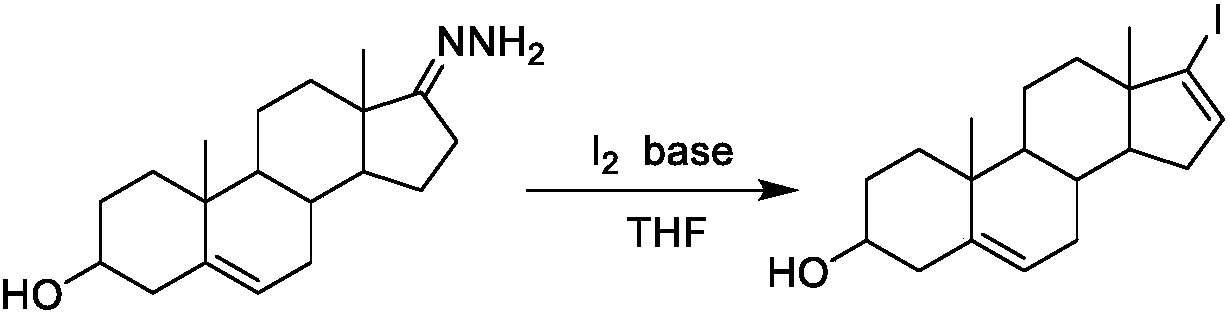
17-Iodoandrosta-5,16-dien-3beta-ol preparation method
A technology of androstane and diene is applied in the directions of androstane derivatives, chemical instruments and methods, steroids, etc., which can solve the problem of large amount of iodine element, achieve simple operation, good industrial application prospects, and reduce the generation of impurities amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Add 0.84g of simple iodine, dissolve in 80ml of anhydrous ether, cool down to 0°C, slowly add 0.89g of TCCA, add 1.95g of tetramethylguanidine dropwise at 0°C, stir evenly, start to dropwise add 1.02g of dehydrogenated form Androsterone-17-hydrazone tetrahydrofuran (35ml) solution, control the reaction temperature -5-5 ° C, after the dropwise addition, remove the ice bath, react at room temperature for 1 h, filter the reaction solution, concentrate the filtrate, and obtain the oil under nitrogen protection Heat at 90°C for 4 hours, add an appropriate amount of ethyl acetate to reflux, filter, wash the filtrate with dilute hydrochloric acid, saturated brine, and pure water, collect the organic layer, filter, and concentrate to obtain 1.30 g of a light yellow solid, ethanol: water = 2: 1. Recrystallized to obtain 1.10 g of off-white solid, namely steroidal iodide: 17-iodoandrost-5,16-dien-3β-ol, with a yield of 83%. Example two
example 2
[0022] Add 0.84g of elemental iodine, dissolve in 80ml of anhydrous ether, cool down to 0°C, slowly add 0.89g of TCCA, add 2.65g of MTBD dropwise at 0°C, stir evenly, start to add 1.03g of dehydroepiandrosterone-17 -Hydrazone tetrahydrofuran (35ml) solution, control the reaction temperature -5-5°C, after the dropwise addition, remove the ice bath, react at room temperature for 1h, filter the reaction solution, concentrate the filtrate, and heat the obtained oil at 90°C under nitrogen protection After 4 hours, add an appropriate amount of ethyl acetate to reflux, filter, and wash the filtrate with dilute hydrochloric acid, saturated brine, and pure water respectively, collect the organic layer, filter, and concentrate to obtain 1.20 g of a light yellow solid, recrystallized with ethanol:water=2:1, 0.99 g of an off-white solid was obtained, which was steroidal iodide: 17-iodoandrost-5,16-dien-3β-ol, with a yield of 75%.
example 3
[0024] Add 0.84g of simple iodine, dissolve in 80ml of anhydrous ether, cool down to 0°C, slowly add 0.90g of TCCA, control the temperature at 0°C, add 1.90g of DABCO dropwise, stir evenly, start to drop 1.02g of dehydroepiandrosterone-17 -Hydrazone tetrahydrofuran (35ml) solution, control the reaction temperature -5-5°C, after the dropwise addition, remove the ice bath, react at room temperature for 1h, filter the reaction solution, concentrate the filtrate, and heat the obtained oil at 90°C under nitrogen protection After 4 hours, add an appropriate amount of ethyl acetate to reflux, filter, and wash the filtrate with dilute hydrochloric acid, saturated brine, and pure water respectively, collect the organic layer, filter, and concentrate to obtain 1.26 g of a light yellow solid, recrystallized with ethanol:water=2:1, 1.06 g of off-white solid was obtained, namely steroidal iodide: 17-iodoandrost-5,16-dien-3β-ol, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com