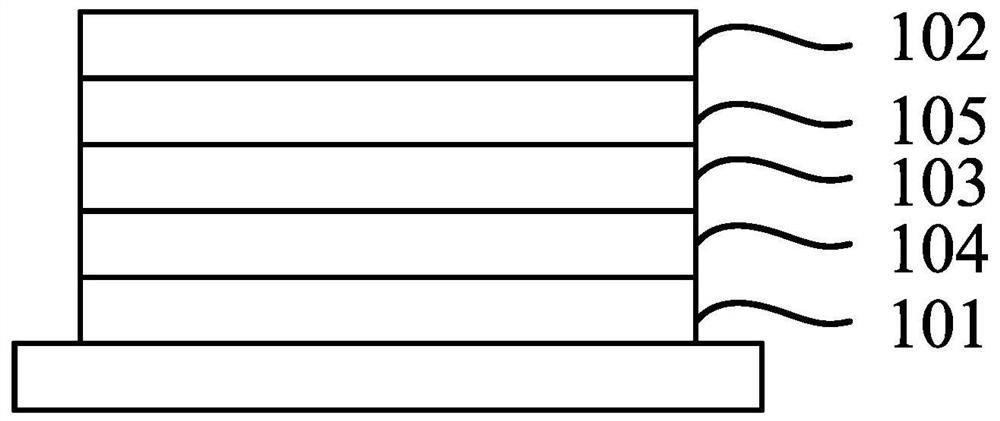
A thermally activated delayed fluorescent material and an organic light-emitting display device comprising the same
A technology of thermally activated delayed and fluorescent materials, applied in the fields of luminescent materials, organic chemistry, silicon organic compounds, etc., can solve the problems of single type of materials, unable to meet the development needs of OLED devices, etc., and achieve the effect of improving luminous efficiency and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0096] Compound P5 is synthesized according to the following route:
[0097]
[0098]Put S1 (10.5mmol), S2 (10mmol), (dibenzylideneacetone) dipalladium (0) (0.05mmol), sodium tert-butoxide (14mmol), tert-butylphosphine (0.2mmol) into a 100mL three-necked flask , while stirring, while rapidly repeating degassing and nitrogen replacement 3 times, 20 mL of toluene was added through a syringe. The mixture was heated to reflux for 12 hours under a stream of nitrogen. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain intermediate P5 (8.2 mmol, yield 82%).
[0099] Carry out elemental analysis structure to P5 (molecular formula C 28 h 29 NO 2 S): Calculated: C, 75.81; H, 6.59; N, 3.16; O, 7.21; S, 7.23; Found: C, 75.83; H, 6.61; N...
preparation example 2
[0101] Compound P8 is synthesized according to the following route:
[0102]
[0103] Specific steps: S3 (8.5mmol), S4 (8mmol), (dibenzylideneacetone) dipalladium (0) (0.06mmol), sodium tert-butoxide (12mmol), tert-butylphosphine (0.3mmol) into 100mL In the three-necked flask, while stirring, degassing and nitrogen replacement were repeated three times rapidly, and 20 mL of toluene was added through a syringe. The mixture was heated to reflux for 12 hours under a stream of nitrogen. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain product P8 (5.76 mmol, yield 72%).
[0104] Carry out elemental analysis structure to P8 (molecular formula C 39 h 26 N 2 o 2 S): Calculated: C, 79.84; H, 4.47; N, 4.77; O, 5.45; S, 5.47; Found:...
preparation example 3
[0106] Compound P14 is synthesized according to the following route:
[0107]
[0108] Specific steps: put S5 (6.0mmol), S6 (6.3mmol), (dibenzylideneacetone) dipalladium (0) (0.05mmol), sodium tert-butoxide (10mmol), tert-butylphosphine (0.25mmol) into In a 100mL three-neck flask, while stirring, degassing and nitrogen replacement were repeated 3 times rapidly, and 30mL of toluene was added through a syringe. The mixture was heated to reflux for 12 hours under a stream of nitrogen. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain the target product P14 (4.56 mmol, yield 76%).
[0109] Carry out elemental analysis structure to P14 (molecular formula C 32 h 23 NO 2 SSi): Calculated: C, 74.82; H, 4.51; N, 2.73; O, 6.23; S, 6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com