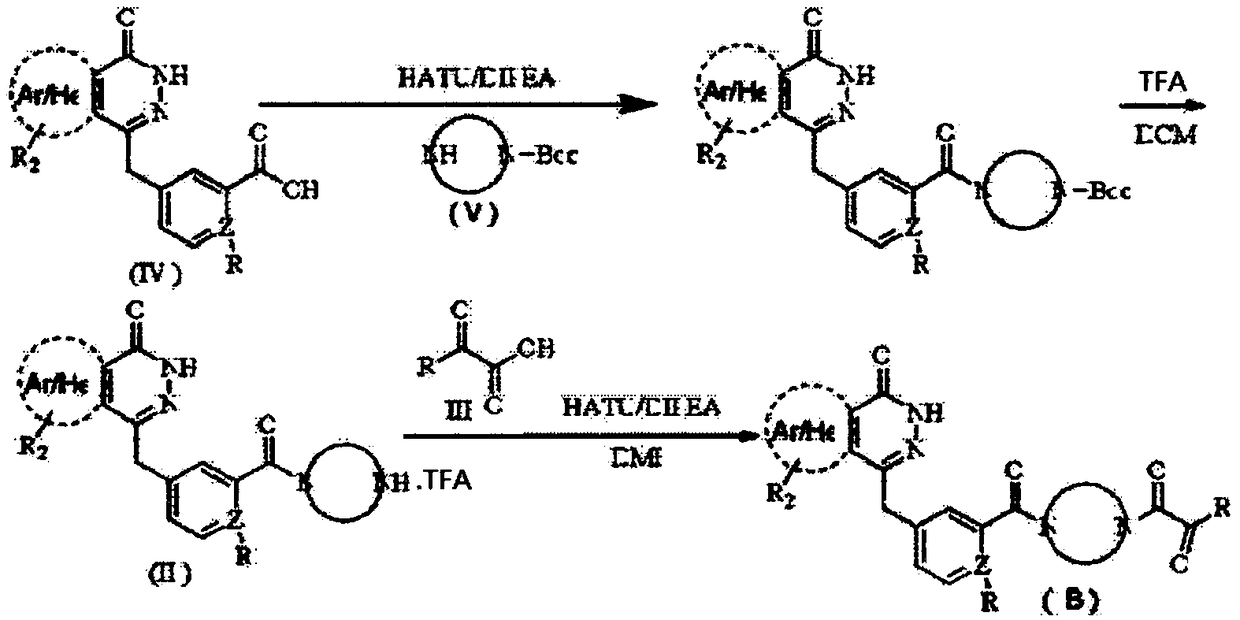
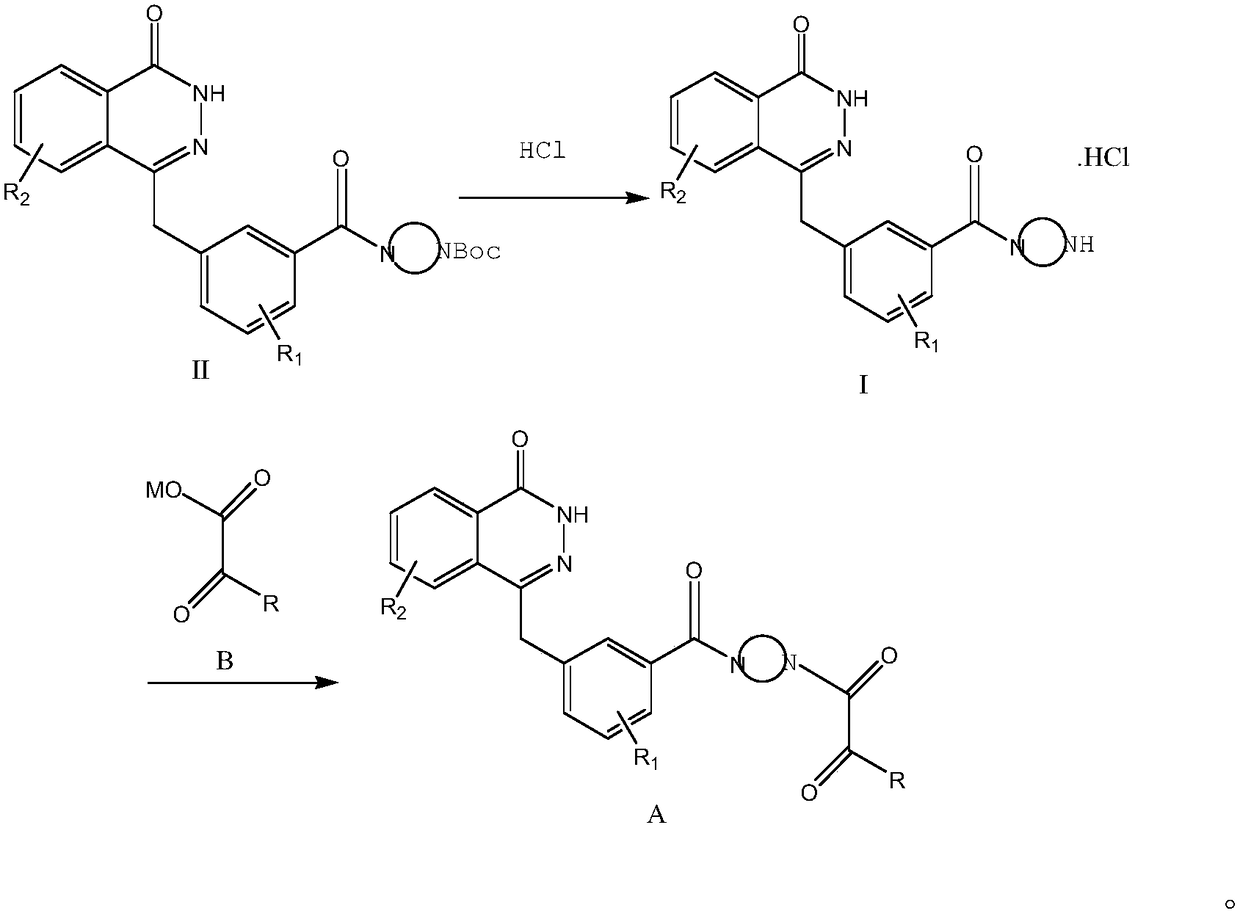
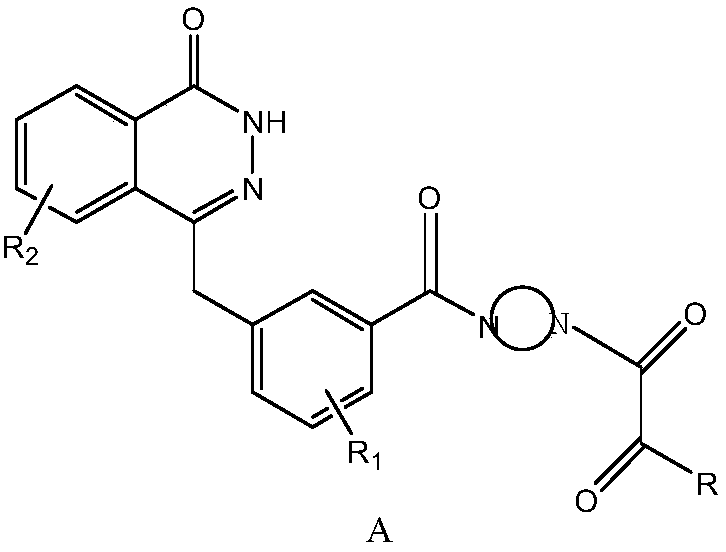
Preparation method and intermediate of compound for inhibiting activity of PARP
A compound and active technology, applied in the field of compound preparation, can solve the problems of no industrial production, low purity and yield of compound B, difficult purification and quality control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1: (1S,4S)-5-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)-2,5- Preparation of tert-butyl diazabicyclo[2,2,1]heptane-2-carboxylate
[0107] Add dichloromethane (700ml), 2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid (70g, 0.24mol) to the reaction flask successively, (1S,4S)-2,5-Diazabicyclo[2,2,1]heptane-2-carboxylic acid tert-butyl ester (71.4g, 0.36mol) and 2-(7-azobenzotriazole )-N,N,N',N'-tetramethyluronium hexafluorophosphate (136.5g, 0.36mol), stirring. N,N-diisopropylethylamine (70.0 g, 0.54 mol) was added dropwise, stirred at room temperature for 2 hours, and the reaction of raw materials was monitored by TLC to complete. Add 700ml of water, stir, separate the liquids, wash the organic phase, dry over anhydrous sodium sulfate, filter, and concentrate to obtain a brown oil with a mass of 157.2g.
Embodiment 2
[0108] Example 2: (1S,4S)-5-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)-2,5- Preparation of tert-butyl diazabicyclo[2,2,1]heptane-2-carboxylate
[0109] Add 500ml of dichloromethane, 2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid (70g, 0.24mol), (1S ,4S)-tert-butyl 2,5-diazabicyclo[2,2,1]heptane-2-carboxylate (71.4 g, 0.36 mol), stirred. 1-Hydroxybenzotriazole (HOBT) (48.6 g, 0.36 mol), 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (EDCI) (69.0 g , 0.36mol). The temperature was controlled at 10°C, and triethylamine (36.4g, 0.36mol) was added dropwise. After the dropping was completed, the reaction was stirred at a temperature of 10°C for about 4 hours, and the reaction of the raw materials was monitored by TLC. Add 500ml of water, stir, separate, wash the organic phase, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate at 40°C under reduced pressure to obtain a white solid with a mass of 148g.
Embodiment 3
[0110] Example 3: (1S,4S)-5-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)2,5-di Preparation of tert-butyl azabicyclo[2,2,1]heptane-2-carboxylate
[0111] Add 500ml of dichloromethane, 2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid (70g, 0.24mol), (1S ,4S)-tert-butyl 2,5-diazabicyclo[2,2,1]heptane-2-carboxylate (47.6 g, 0.24 mol), stirred. HOBT (39.2 g, 0.29 mol) was added followed by EDCI (55.6 g, 0.29 mol). The temperature was controlled at 10°C, and N,N-diisopropylethylamine (37.6 g, 0.29 mol) was added dropwise. After dropping, the temperature was controlled at 40° C. and the reaction was stirred for about 24 hours. TLC monitored the complete reaction of the raw materials. Add 500ml of water, stir, separate liquids, wash the organic phase, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate at 40°C under reduced pressure.
[0112] A white solid with a mass of 152 g was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com