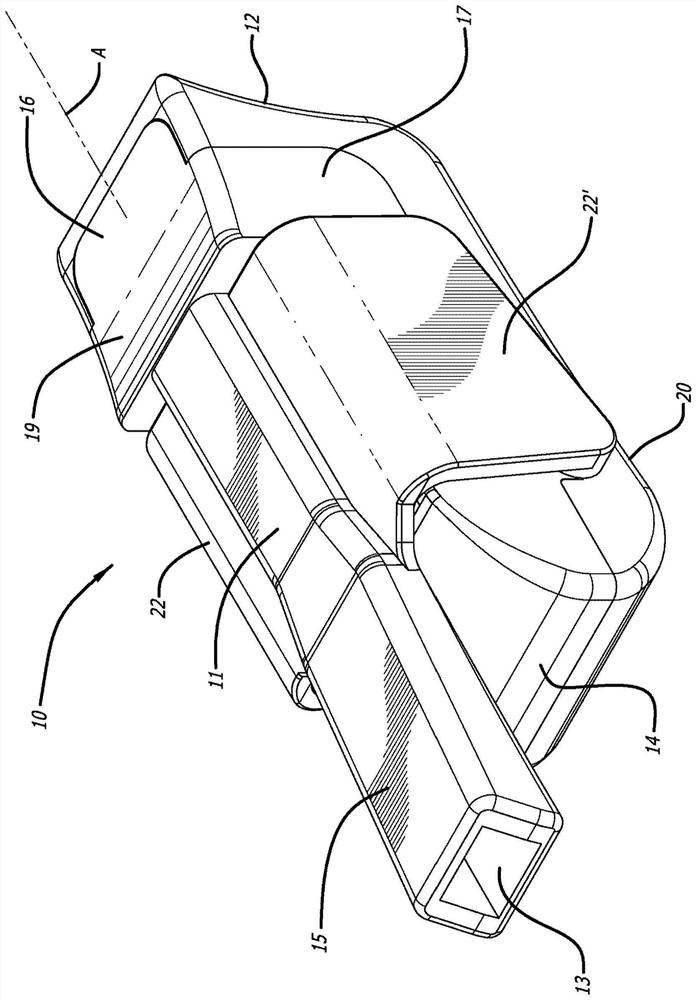
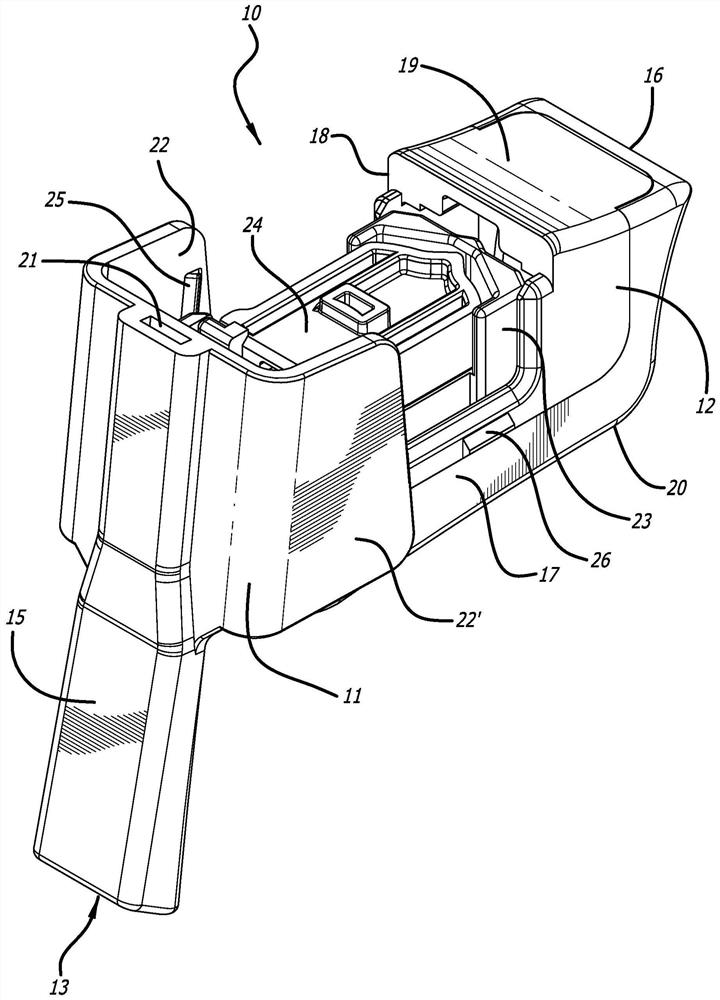
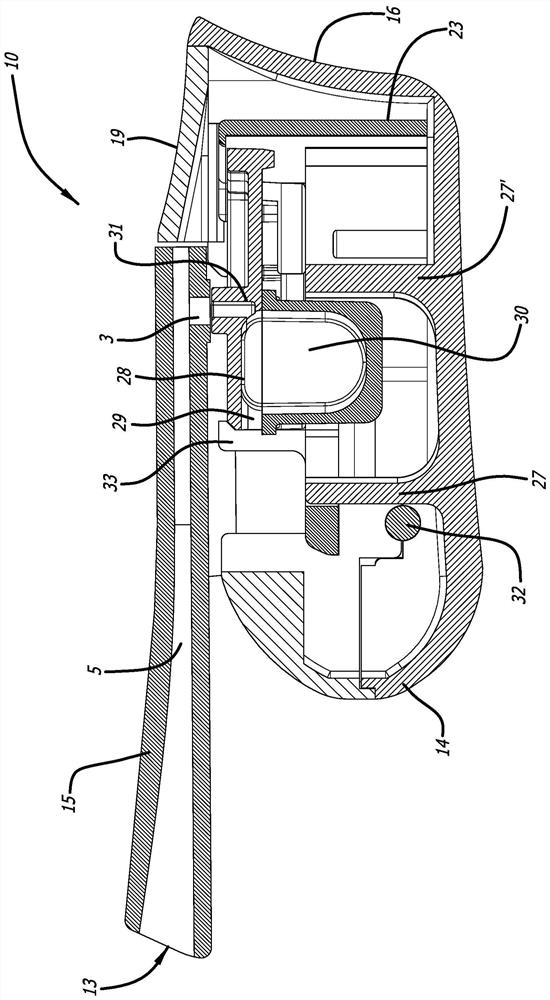
dry powder inhaler
A technology of dry powder inhaler and inhaler, which is applied in the direction of inhaler, powder delivery, drug equipment, etc., and can solve the problems of lack of equipment durability, lack of practicability, manufacturing cost, poor depolymerization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0119] Preparation of a surfactant-free dry powder comprising FDKP unpowder for inhalers: In an exemplary embodiment, a surfactant-free dry powder comprising FDKP microcrystalline particles was prepared. Using a dual-wire fed high shear mixer, feed approximately equal masses of acetic acid solution (Table 1) and FDKP solution (Table 2) maintained at approximately 25 °C ± 5 °C through a 0.001-in2 orifice at 2000 psi to A precipitate is formed by homogenization. The precipitate was collected in deionized (DI) water of approximately equal temperature. The weight percent content of FDKP crystallites in the suspension is about 2-3.5%. The FDKP concentration of the suspension can be analyzed by drying method for solids content. The FDKP microcrystalline suspension can optionally be washed by tangential flow filtration using deionized water. FDKP crystallites can optionally be isolated by filtration, concentration, spray drying or lyophilization.
[0120] Table 1 Composition of F...
example 2
[0129] Preparation of dry powders including microcrystalline FDKP particles containing epinephrine. To the FDKP microcrystalline suspension obtained as described in Example 1 was added approximately 5% by weight of epinephrine in approximately 5% aqueous acetic acid. Optionally, leucine was also added to the FDKP microcrystalline suspension. The mixture was spray dried using a Buchi B290 spray dryer equipped with a high efficiency cyclone. Nitrogen was used as process gas (60mm). The mixture was dried using 10%-20% pump flow, 90%-100% suction speed and inlet temperature of 170-190°C. The weight percent concentrations of epinephrine and leucine in the resulting powder were 2%-30% and 0%-20%, respectively. The delivery efficiencies of these powders after expulsion from the dry powder inhaler ranged from approximately 50% to 80%.
example 3
[0131] Preparation of dry powders including microcrystalline FDKP particles containing palonosetron. To the FDKP microcrystalline suspension obtained as described in Example 1 was added approximately 5% by weight of a solution of palonosetron hydrochloride in DI. Optionally, a solution of leucine and methionine in deionized water (DI). The mixture was titrated to pH 6.5±0.5 with ammonium hydroxide. The mixture was spray dried using a Buchi B290 spray dryer equipped with a high efficiency cyclone. Nitrogen was used as process gas (60mm). The mixture was dried using 10%-12% pump flow, 90%-100% suction speed and inlet temperature of 170-190°C. The weight percent concentrations of palonosetron, leucine, and methionine in the resulting powder were 5%, 0%-20%, and 0%-10%, respectively. The delivery efficiencies of these powders after expulsion from the dry powder inhaler ranged from approximately 50% to 70%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com