Asthma treating pharmaceutical composition
A composition and drug technology, applied in the field of medicine, can solve the problems of poor patient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0021] The characterization of experimental example 1 medicine of the present invention
[0022]
[0023] 1 H NMR (400MHz, CDCl 3 ):δ10.85(s,1H),9.86(s,1H),8.02(d,J=13.1Hz,1H),6.65(dd,J=8.7,7.7Hz,2H),4.53(d,J= 3.9Hz, 2H), 4.40(t, J=5.1Hz, 1H), 4.18(s, 2H), 3.42(s, 6H), 3.39(m, 2H), 3.37(s, 3H).
[0024] 13 C NMR (100MHz, CDCl 3 ): δ193.30, 169.15, 162.10 (ddd, J = 248.9, 15.5, 15.5Hz), 161.7 (ddd, J = 250.0, 14.9, 11.1Hz), 161.66, 111.08 (ddd, J = 19.9, 19.9, 4.7Hz) 103.12 ,100.29(ddd,J=28.1,17.7,2.3Hz),76.30,58.83,54.98,53.53,51.57,29.89(t,J=3.3Hz).
experiment example 2
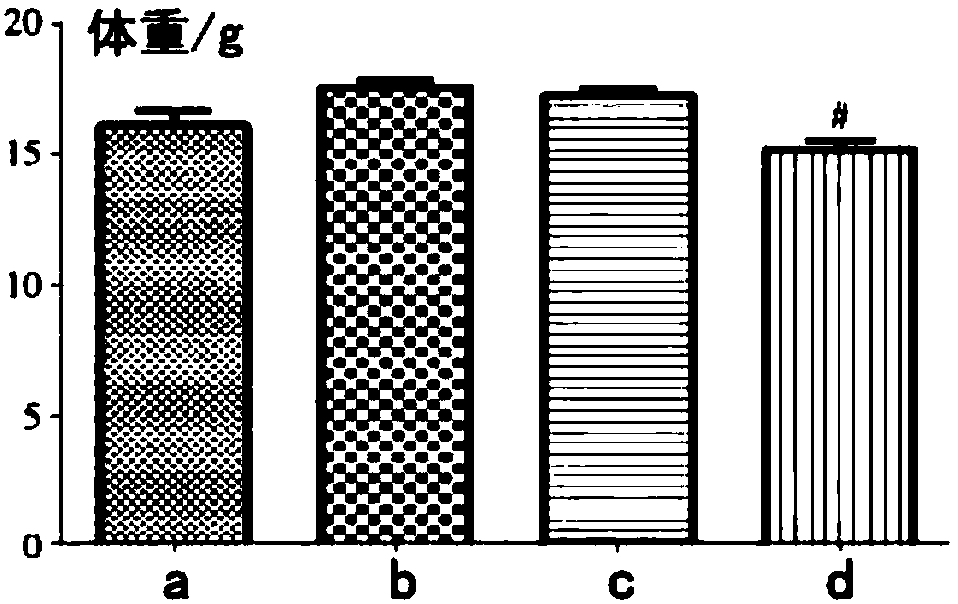
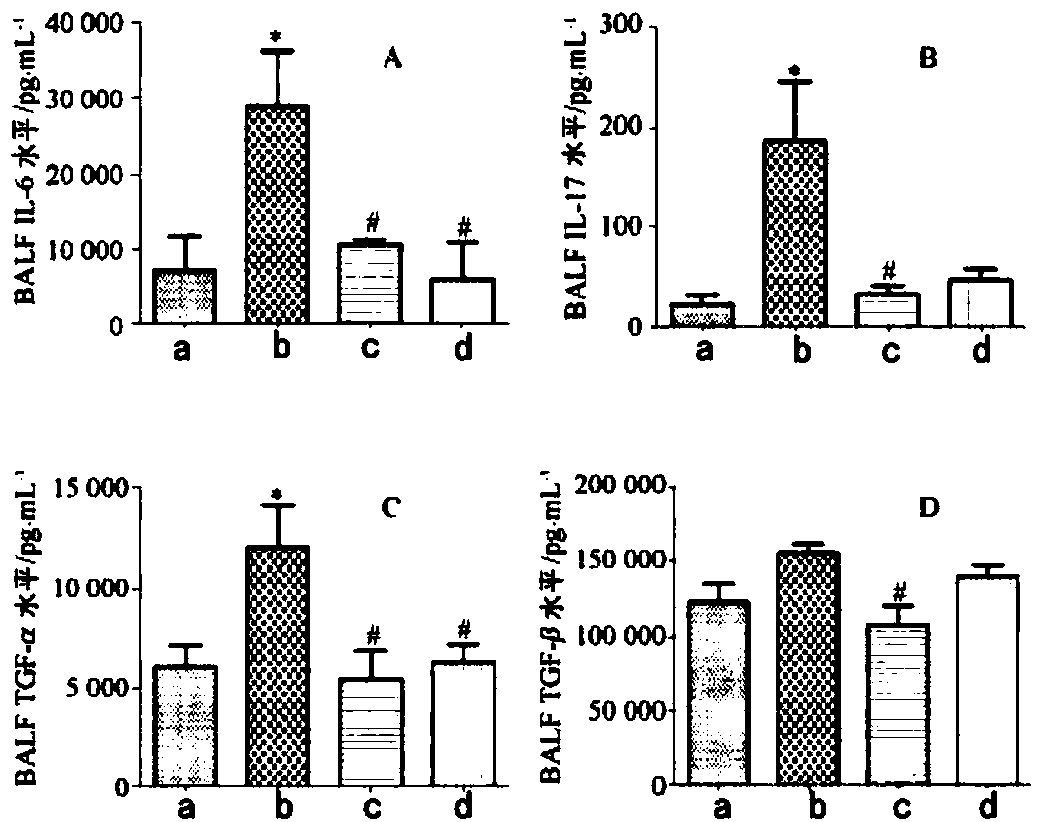
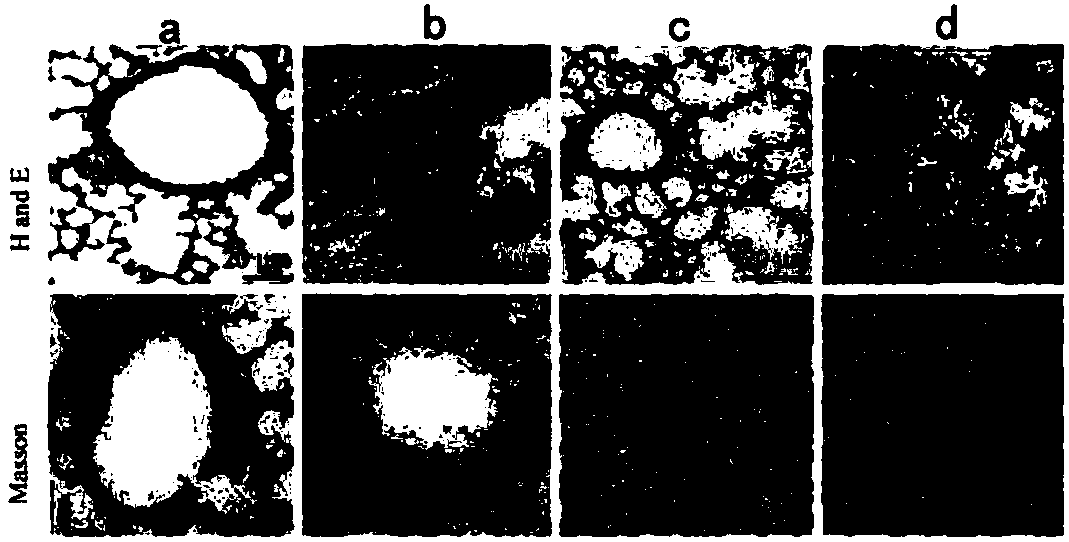
[0025] Experimental example 2 The therapeutic effect of medicine of the present invention for asthma
[0026] Sixty healthy female Kunming mice were randomly divided into a normal group of 10 and a model group of 30 after weighing.
[0027] Preparation of Mouse Allergic Asthma Model
[0028] Mice in the model group were induced by mites (HDM) as a mouse allergic asthma model: after mixing 25 μg of crude HDM extract with 10 μL of 0.9% NaCl, each mouse was intranasally instilled with 10 μL each time, 5 times a week, for 7 consecutive days. week. The mice in the normal group were intranasally instilled with 10 μL of normal saline, and the procedure was the same as above.
[0029] Animal grouping and dosing
[0030] The model mice were divided into a model group, a positive control group and a drug group of the present invention, with 10 mice in each group.
[0031] After 5 weeks of sensitization, the mice of the drug group of the present invention were administered with 10 mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com