Application of 25-hydroxyvitamin D in preparation of disease activity evaluation kit for patients with Takayasu arteritis
A technology of hydroxyvitamins and Takayasu arteritis, which is applied in the field of preparation of disease activity evaluation kits for patients with Takayasu arteritis, can solve the problems of being unable to meet the clinical needs of accurately judging disease activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
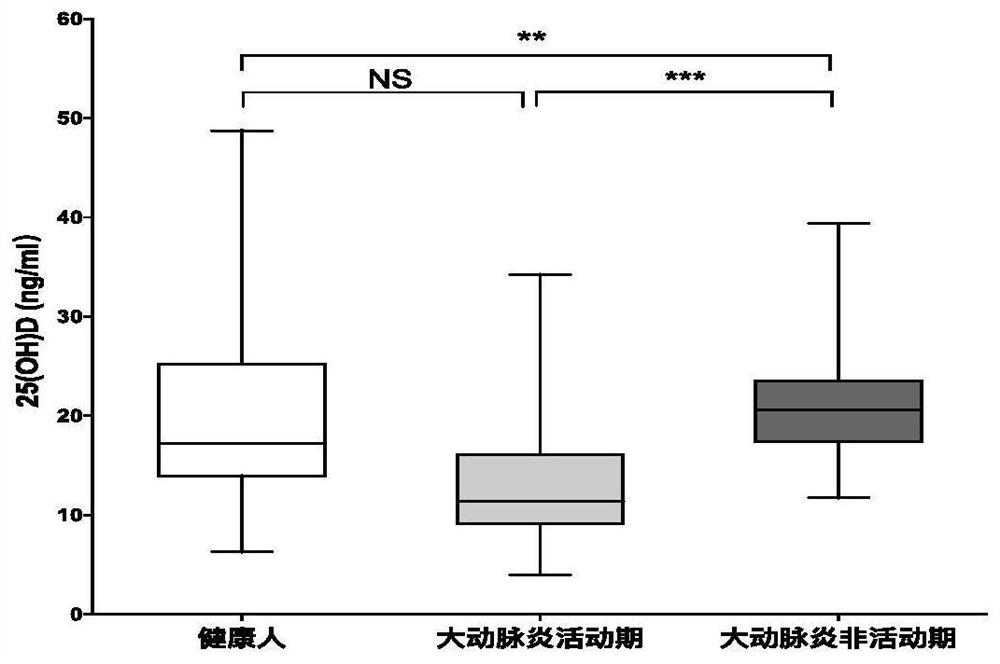
[0052] By detecting the concentration of 25(OH)D in the plasma of 126 patients with Takayasu arteritis and 154 healthy control subjects, the mean value of 25(OH)D in the plasma of 154 healthy control groups was 19.63ng / ml (range: 8.79 to 36.64ng / m1), and the level of plasma 25(OH)D in patients with Takayasu arteritis was significantly lower than that in healthy controls (p<0.01), and the average value was 16.29 (the numerical value here is to include active Takayasu arteritis patients and latent period Mean values obtained from patients with Takayasu arteritis) ng / ml (range: 5.2 to 39.40 ng / m1).
[0053] When patients with Takayasu arteritis were divided into active phase and latent phase according to disease activity, the mean value of plasma 25(OH)D in active phase patients was 13.29 ng / ml (range: 5.2 to 30.05 ng / m1), while in The average value of patients in the incubation period was 21.25ng / ml (range: 14.92 to 39.40ng / m1), and the results of statistical analysis showed t...
Embodiment 2
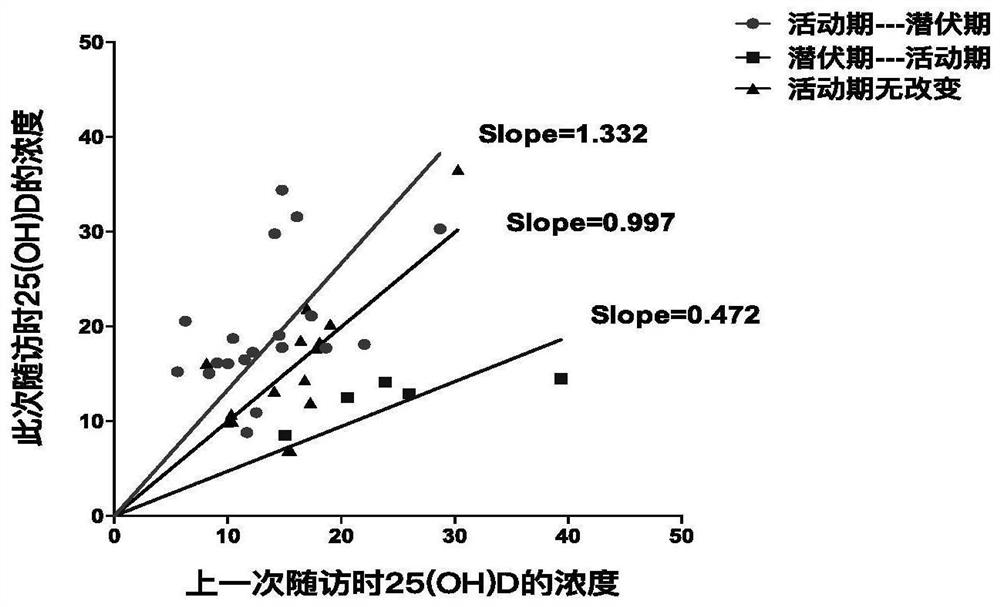
[0060] In the treatment of patients with Takayasu arteritis, commonly used drugs are glucocorticoids and immunosuppressants. Hormone is still the main treatment drug for patients with active disease, but hormone therapy can cause adverse reactions such as osteoporosis, so patients using hormone therapy will be given vitamin D supplementation. So does vitamin D supplementation affect the association between 25(OH)D and disease activity? The present study found that vitamin D supplementation or no supplementation did not affect the correlation between 25(OH)D and disease activity. Such as figure 2 It was shown that whether the patients with Takayasu arteritis were supplemented with vitamin D or not, when the disease activity turned into the latent stage, the plasma 25(OH)D level rose to a cutoff value of 15.98ng / ml or more. When the active state of the disease turned to active, the 25(OH)D level decreased to below the cutoff value of 15.98ng / ml.
[0061] During the treatment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com