A biomarker composition for non-small cell lung cancer, and screening and applications of the biomarker composition
A non-small cell lung cancer and biomarker technology, applied in the field of molecular medical diagnosis, can solve the problems of high tumor mortality, missing the best treatment time, lack of early diagnosis technology, etc., to achieve high prediction accuracy, early detection opportunity, Improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1. Preliminary screening of differentially expressed miRNAs in non-small cell lung cancer
[0047] In this embodiment, the following steps are included:
[0048] (1) The goal of the present invention is to systematically study the relationship between circulating miRNAs and non-small cell lung cancer, in order to find a group of miRNAs that can be used as biomarkers for early diagnosis of non-small cell lung cancer. Firstly, the range of candidate research miRNAs was determined by literature search, and the search keywords were "microRNA / miRNA" and "cancer". A total of 486 miRNAs were identified as candidate research targets (Table 1).
[0049] (2) Preparation of mixed samples. All samples (from Shenzhen People's Hospital) were divided into three groups, including 266 healthy people's plasma, 130 non-small cell lung cancer stage I patients' plasma and 158 II-IV stage patients' plasma, which were mixed evenly.
[0050] (3) extract plasma total RNA, use S / P miR...
Embodiment 2
[0066] Example 2. Re-screening differentially expressed miRNAs in non-small cell lung cancer
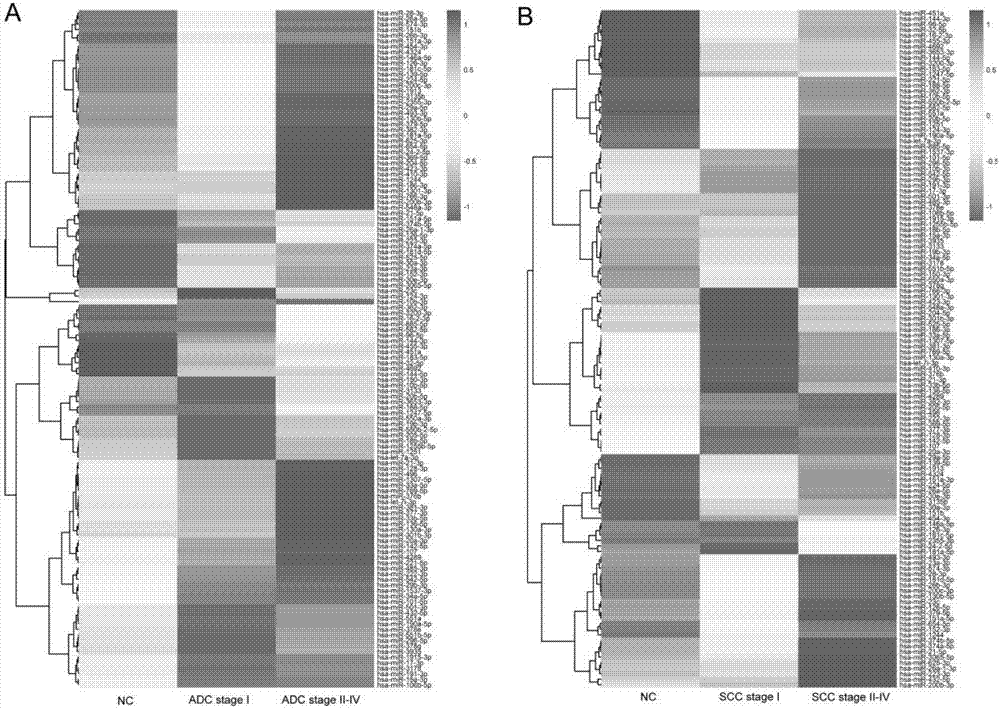
[0067] In the second round of screening, the specific experimental operations were the same as in Example 1. 544 plasma samples from Shenzhen People's Hospital were divided into five groups: NC (N=266), ADC Stage I (N=96), ADC Stage II-IV (N=113), SCCStage I (N=34 ) and SCC Stages II-IV (N=45). The expression levels of the 125 miRNAs screened out in Example 1 were detected in five groups of mixed samples respectively. The selection criteria for this round of screening are: Stage I vs. NC: fold-change>2, or Stage II-IV vs. NC: fold-change>2. There were 30 miRNAs meeting the criteria in adenocarcinoma and 38 in squamous cell carcinoma ( Figure 4 ).
Embodiment 3
[0068] Example 3, adenocarcinoma / squamous cell carcinoma miRNA biomarker single verification pilot experiment
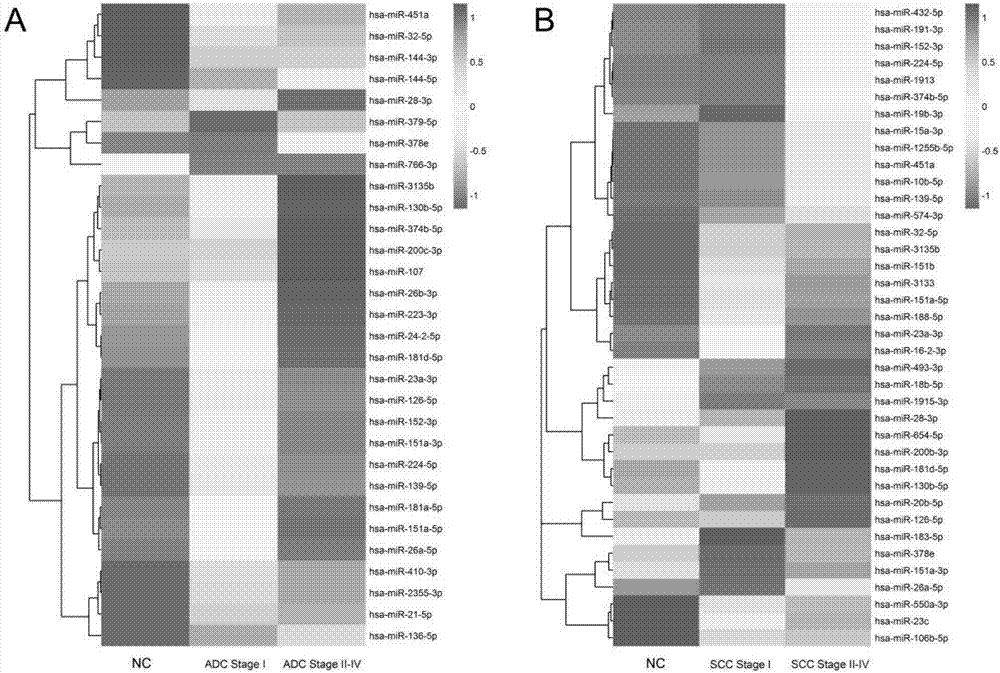
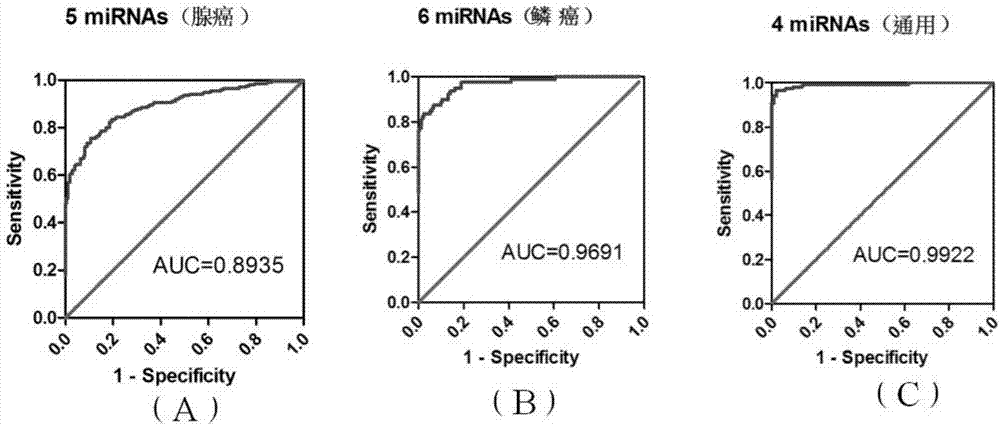
[0069] The miRNAs screened in Example 2 were firstly verified with a small number of random samples (collected in Shenzhen People's Hospital). The number of selected samples is: healthy group (NC) (N=20), adenocarcinoma stage I (N=20), adenocarcinoma stage II-IV (N=20), squamous cell carcinoma stage I (N=10) and Squamous cell carcinoma stage II-IV (N=10). The selection criteria for this round of screening are: Phase I vs. healthy group: fold-change>2, or Phase II-IV vs. healthy group: fold-change>2. There were 11 miRNAs meeting the criteria in adenocarcinoma and 10 in squamous cell carcinoma ( Figure 5 ). Among them, hsa-miR-32-5p, has-miR-183-5p, hsa-miR-144-5p, has-miR-144-3p and hsa-miR-574-3p are Ct in some adenocarcinoma and squamous cell carcinoma samples Values greater than 35 are discarded. Therefore, in this example, 7 miRNAs (adenocarcinoma) and 9 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com