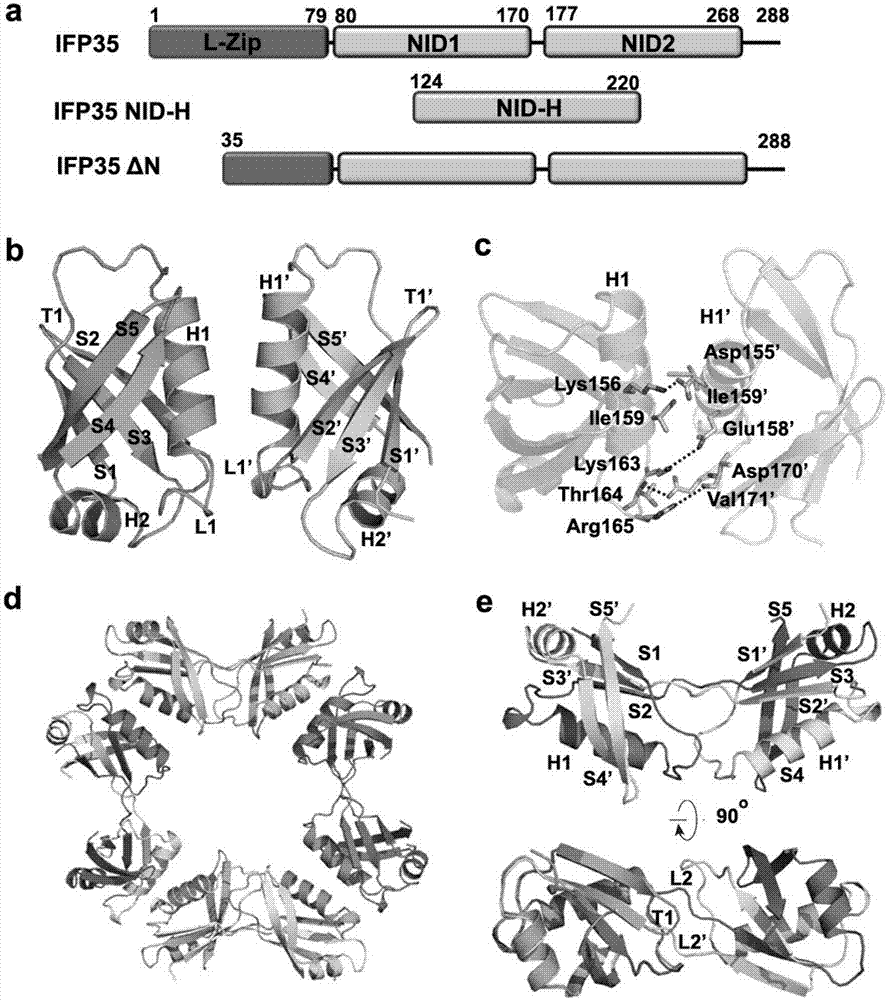
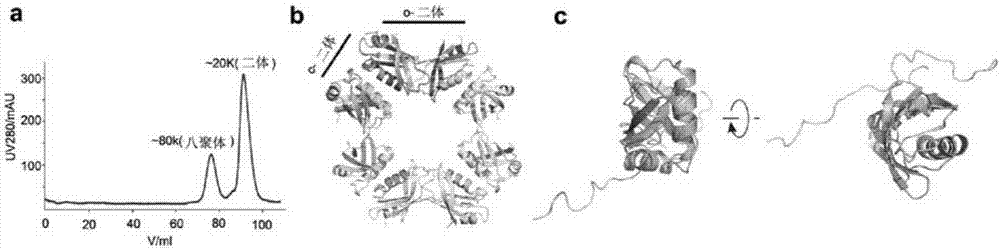
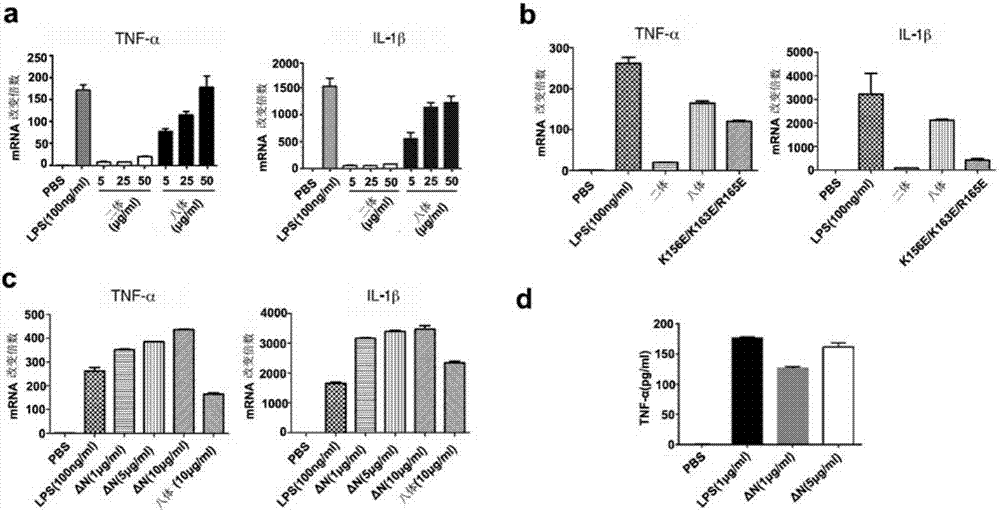
Methods and compositions for treating and/or preventing a disease or disorder associated with abnormal level and/or activity of the IFP35 family of proteins
一种活性、疾病的技术,应用在过敏性疾病、肽的制备方法、肽/蛋白质成分等方向,能够解决蛋白质功能机制不清楚等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0136] Embodiment 1: Use of truncated IFP35 protein in reagents / products that cause immune response and inflammation or in the preparation of anti-tumor products, wherein the truncated IFP35 is human IFP35 or mouse IFP35, wherein the truncated human IFP35 is the following 1) or 2).
[0137] 1) a protein having residues 136-216 as in SEQ ID NO:2;
[0138] 2) The protein sequence as in 1), wherein 1-10 additional residues are added to the amino or / and carboxyl termini of the sequence, and the sequence homology to the sequence of 1) is at least about 90%.
[0139] wherein said truncated mouse IFP35 is the following 3) or 4):
[0140] A protein having residues 134-216 as in SEQ ID NO:4;
[0141] 4) The protein sequence as in 3), wherein 1-10 additional residues are added to the amino or / and carboxyl termini of the sequence, and the sequence homology to the sequence of 3) is at least about 90%.
Embodiment approach 2
[0142] Embodiment 2: The method of Embodiment 1, wherein the truncated human IFP35 protein is represented by residues 124-220 in SEQ ID NO:2.
Embodiment approach 3
[0143] Embodiment 3: A method for preparing immune response / inflammatory products triggered by IFP35 protein or preparing anti-tumor / cancer products, wherein the IFP35 protein sequence is shown in SEQ ID NO:2 or SEQ ID NO:4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com