Methods of treating diarrhea in companion animals
A companion animal, animal technology, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of premature death, effective or possibly ineffective, insufficient or timely response, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] Preparation of proanthocyanidin polymer compositions and preparations
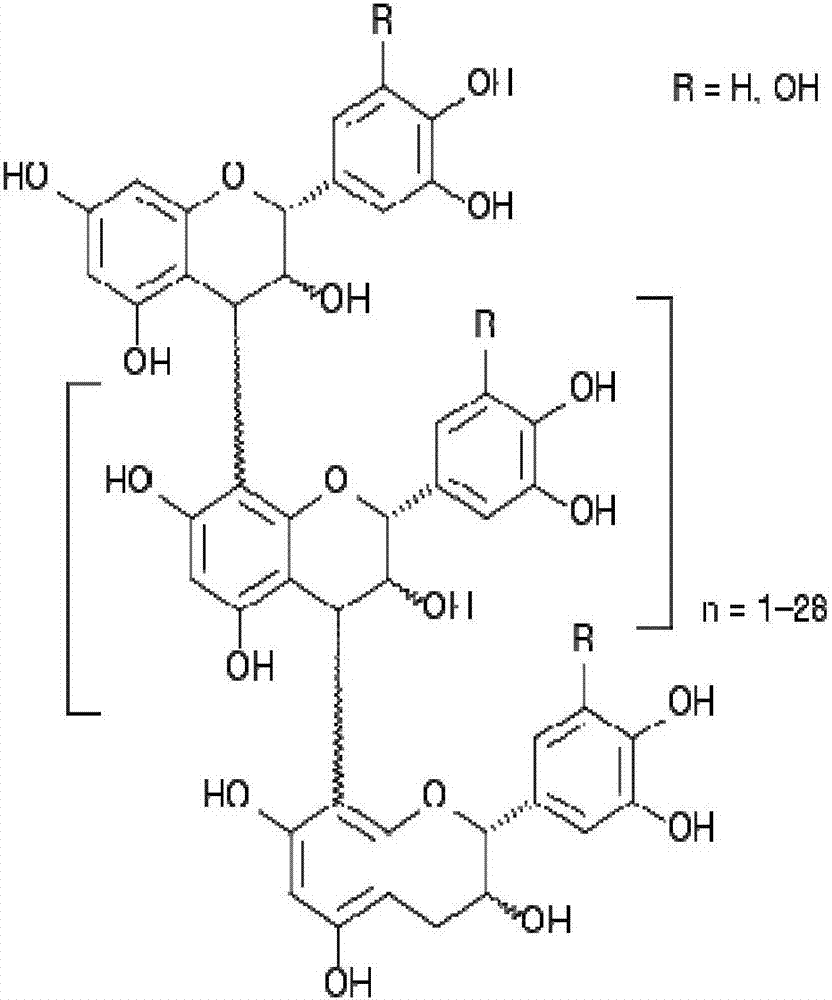
[0062] The proanthocyanidin polymer composition effective for treating secretory diarrhea according to the present invention consists of or includes monomeric units of leucoanthocyanidin. More specifically, the composition is composed of flavonoid units having 2 to 30 flavonoid units, preferably 2 to 15 flavonoid units, more preferably 2 to 11 flavonoid units, most preferably an average of 7 to 8 flavonoid units Proanthocyanidin polymer composition (number average molecular weight about 2500 Da). The proanthocyanidin polymer composition is preferably dissolved in an aqueous solution. Preferred for use in the method according to the invention are proanthocyanidin polymers from Peruvian croton; such Peruvian croton proanthocyanidin polymers may be in the form of a pharmaceutically acceptable composition.
[0063] Examples of the proanthocyanidin polymer composition used in the present invention are ...
Embodiment 1
[0105] Evaluation of Oral Administration of the Peruvian Croton Proanthocyanidin Polymer Composition Crolam (SP 303) to Dogs
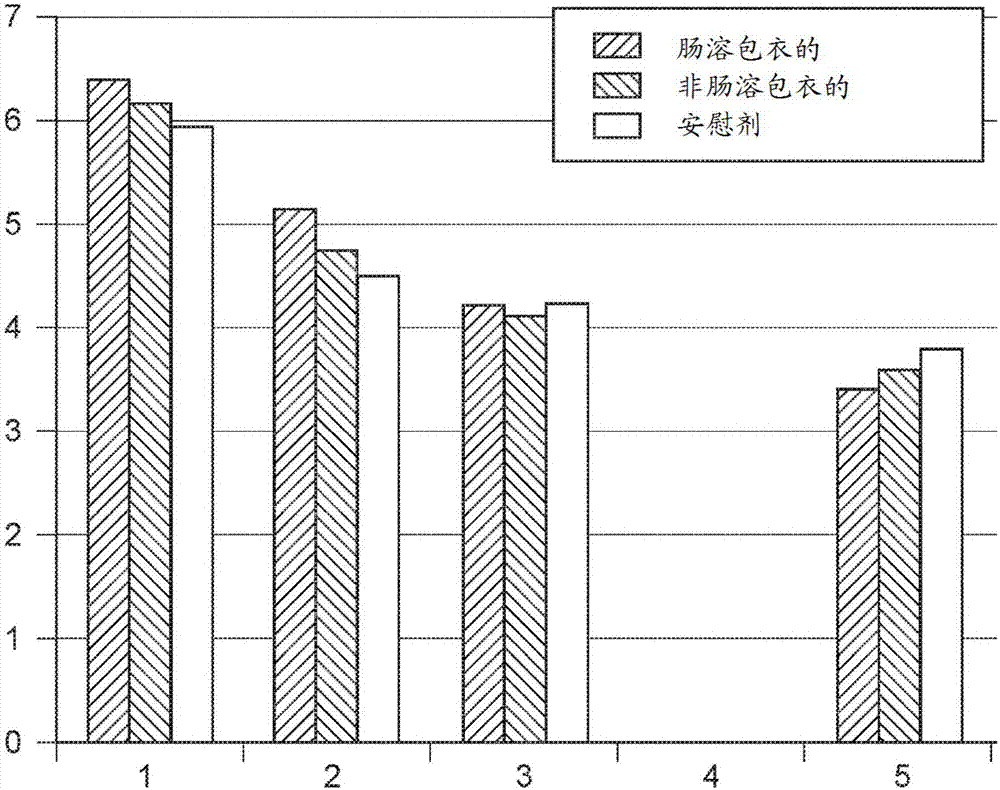
[0106] Described in this Example is a multicenter proof-of-concept study of dogs with acute watery diarrhea. The study was performed in a veterinary hospital to provide a controlled environment. The study was a double-blind randomized block format used to compare five different treatment groups based on cause of diarrhea with each of five relevant placebo groups and for global analysis. The study included recruitment of dogs presenting with usual acute watery diarrhea of less than 3 days. A thorough clinical examination was performed on each dog to determine whether the cause of the diarrhea was chemotherapy, bacterial infection, pancreatitis, dietary inadvertence, or Giardia infection. Each of these five causes was considered a subgroup, and dogs with any other cause of diarrhea were excluded from the study.
[0107] The recruited dogs were hospi...
Embodiment 2
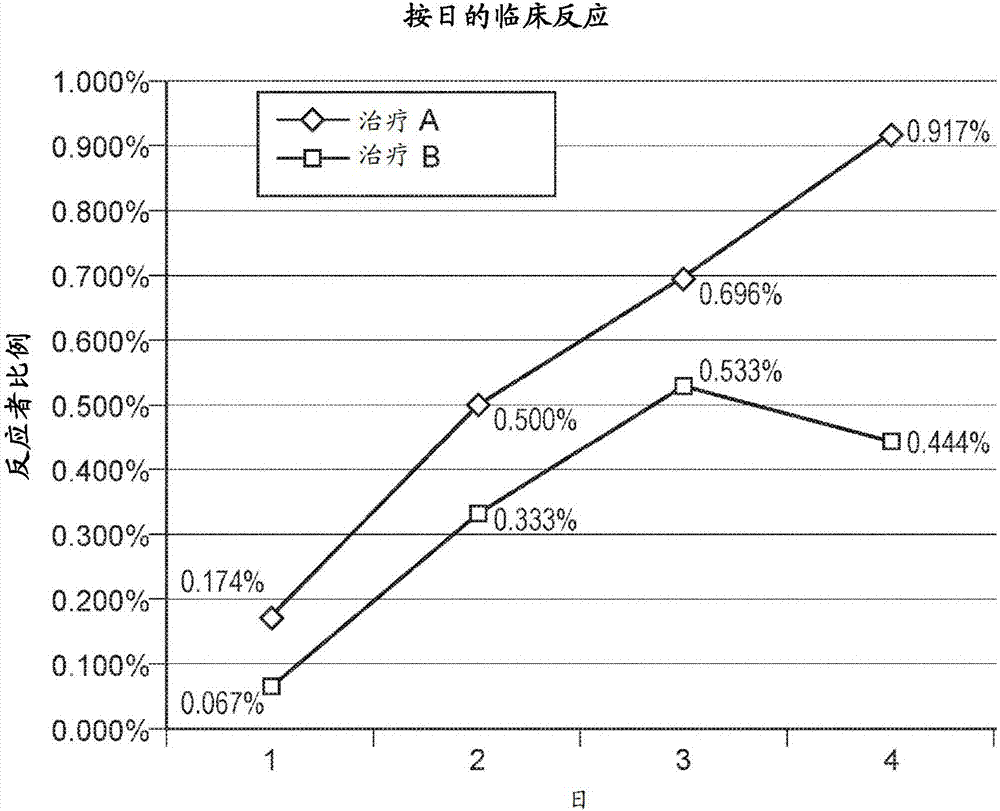
[0110] Evaluation of the Clinical Efficacy of Oral Administration of the Peruvian Croton Proanthocyanidin Polymer Composition Crolam (SP 303) for the Treatment of Diarrhea in Dogs
[0111] This example relates to a blinded randomized controlled study evaluating the clinical efficacy of oral administration of a Peruvian croton proanthocyanidin polymer composition product, Crolam SP303, in the treatment of diarrhea (used by veterinarians through rescue organizations, shelters and customer owners obtained from canines). Investigators were blinded to the treatment task until the end of the study. A minimum of 60 dogs per trial site were recruited over a 4-month period from different veterinary clinics from the same geographical area. Provide financial incentives to rescue organizations, shelters, and owners for recruiting their animals into trials. Animals were given consent to be confined to the study site for a maximum of 6 days. During the first 24 hours of isolation, diarrh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com