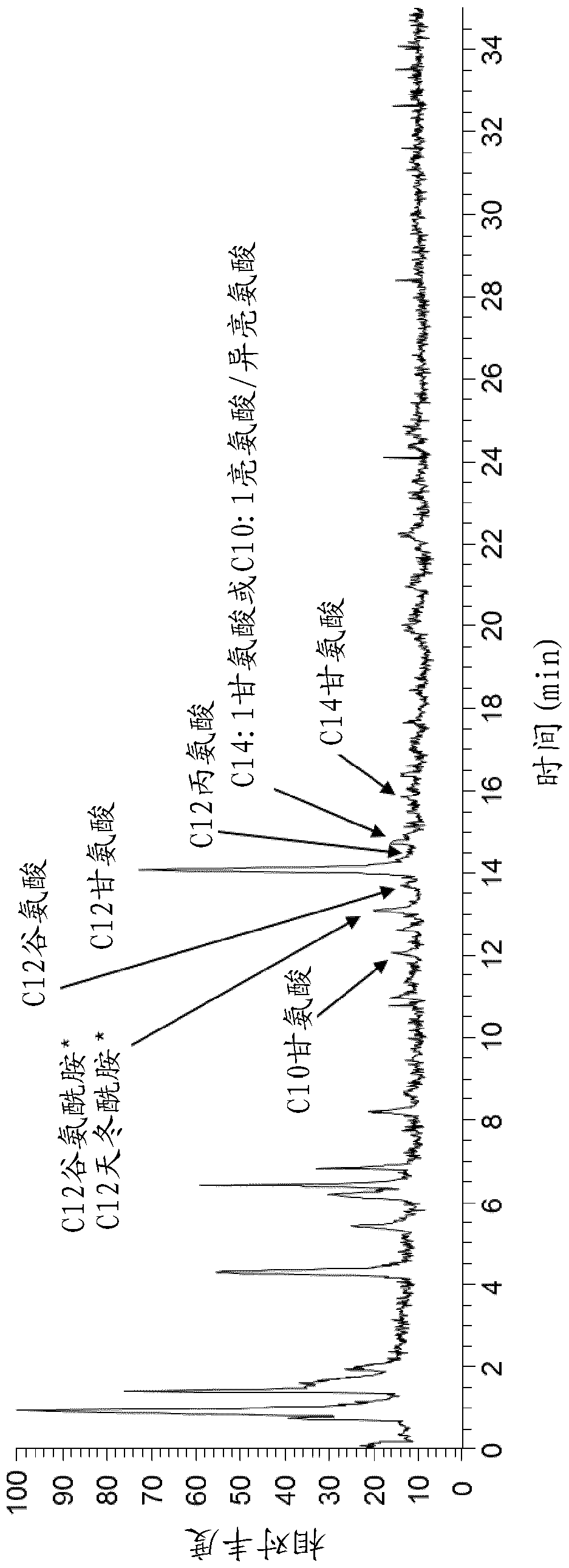
Biosynthetic production of acyl amino acids
An amino acid, a technology for producing acyl glycinate, applied in the directions of microorganism-based methods, microorganisms, acyltransferases, etc., can solve problems such as non-technical feasibility, achieve high-efficiency biotechnology approaches, and improve yield and purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] California Umbrella Gene wxya Preparation of the expression vector
[0163] In order to prepare the california laurel encoding californoyl-CoA-thioesterase wxya The expression vector of the gene (SEQ ID NO: 1), which is codon-optimized for expression in Escherichia coli. The gene and tac The promoter (SEQ ID NO: 2) was synthesized together, and at the same time, a cleavage site was introduced upstream of the promoter and a cleavage site downstream of the terminator. The synthetic DNA fragment P tac -synUcTE with restriction endonuclease Bam H I and not I digested and ligated into correspondingly cut vector pJ294 (DNA2.0 Inc., Menlo Park, CA, USA). The completed E. coli expression vector was named pJ294[Ptac-synUcTE] (SEQ ID NO:3).
Embodiment 2
[0165] Escherichia coli fadD Preparation of co-expression vectors with Homo sapiens genes hGLYAT3 and hGLYAT2
[0166] In order to prepare the Homo sapiens gene hGLYAT2 (SEQ ID NO:4) or hGYLAT3 (SEQ ID NO:5) encoding human glycine-N-acyltransferase and Escherichia coli encoding Escherichia coli acyl-CoA synthetase fadD (SEQ ID NO: 6) co-expression vector, codon-optimized and synthesized genes hGLYAT2 and hGLYAT3 for expression in Escherichia coli. Synthetic DNA fragments were treated with restriction endonucleases Sac II and Eco 47III digested and ligated into the correspondingly cleaved removed aftA 1 gene in pCDF[atfA1_Ab(co_Ec)-fadD_Ec] (SEQ ID NO:7). Sequence segments that would otherwise be removed during this process are co-synthesized during gene synthesis. This vector is a pCDF derivative that already contains a synthetic tac promoter (SEQ ID NO:2) and E. coli fadD Gene. The resulting expression vectors were named pCDF{Ptac}[hGLYAT2(co_Ec)-fadD_Ec] (SEQ I...
Embodiment 3
[0168] Homo sapiens hGLYAT2, Escherichia coli fadD and Pseudomonas putida ( Pseudomonas putida ) Preparation of co-expression vector of alkL gene
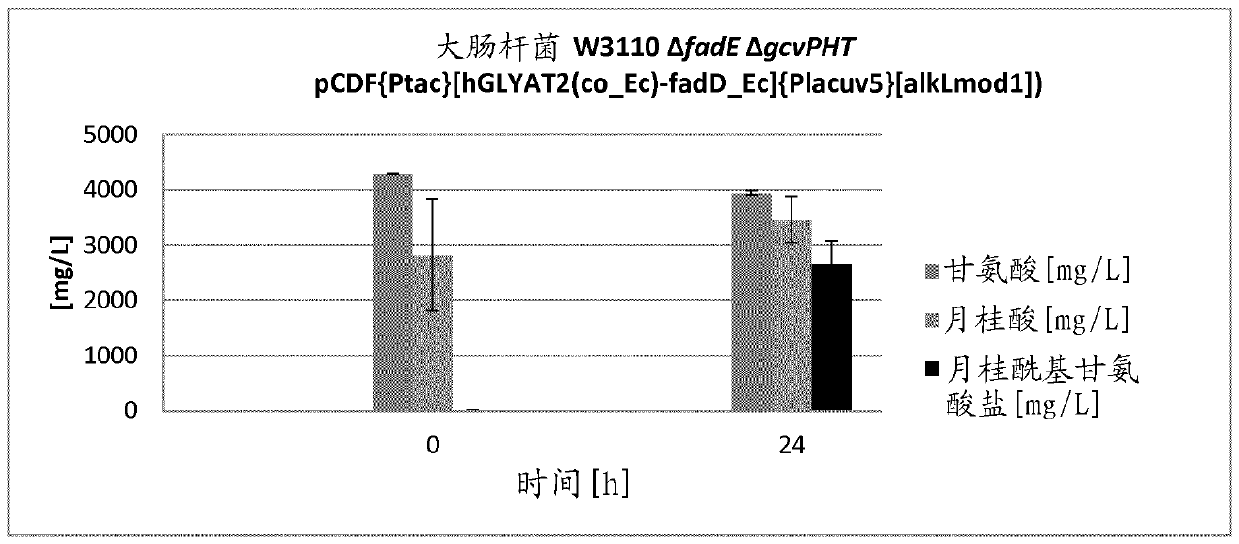
[0169] To prepare a co-expression vector of the hGLYAT2 gene and the modified Pseudomonas putida alkL gene encoding AlkL, an outer membrane protein that facilitates the import of hydrophobic substrates into the cell, with the aid of sequence-specific oligonucleotides acid, the alkL gene (SEQ ID NO: 10) was amplified from plasmid pCDF[alkLmod1] (SEQ ID NO: 12) together with the lacuv5 promoter (SEQ ID NO: 11). restriction endonuclease Bam HI and Nsi I cut and ligated into the correspondingly cut vector pCDF{Ptac}[hGLYAT2(co_Ec)-fadD_Ec] (SEQ ID NO:8). The correct insertion of the target gene is checked by restriction analysis and the authenticity of the introduced gene is verified by DNA sequencing. The resulting expression vector was named pCDF{Ptac}[hGLYAT2(co_Ec)-fadD_Ec]{Placuv5}[alkLmod1] (SEQ ID NO: 13).
[0170] The f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com