A class of 5-aminolevulinic acid derivatives and its preparation method and application
A technology of aminolevulinic acid and its derivatives, which is applied in the field of 5-aminolevulinic acid derivatives and its preparation, can solve the problems of limited cell absorption and high hydrophilicity, achieve strong photodynamic activity, improve fat solubility, and excellent The effect of pharmaceutical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
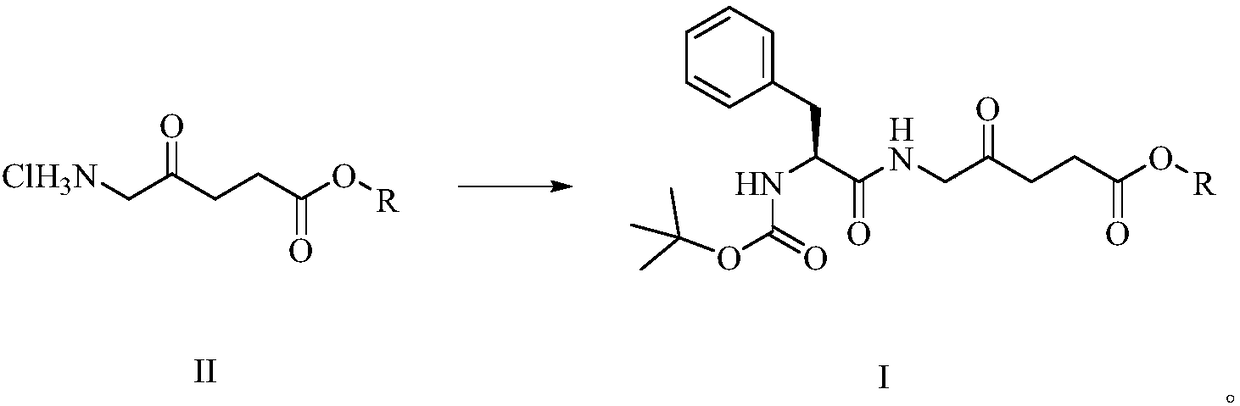
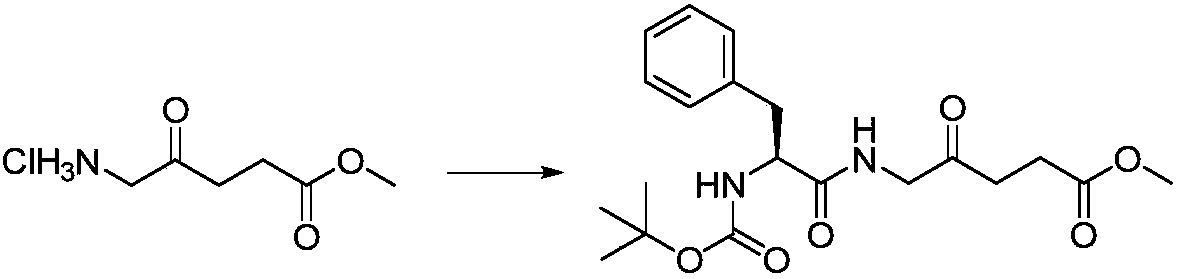
[0023] Preparation of methyl 5-((2'-tert-butyloxycarbonylamino)-L-phenylpropionyl)amino-4-oxopentanoate:
[0024]
[0025] In a 50mL round bottom flask, mix 5-aminolevulinic acid methyl ester hydrochloride (1.0g, 5.5mmol), Boc-L-phenylalanine (1.46g, 5.5mmol), HBTU (1.7g, 4.49 mmol) was dissolved in DMF (10 mL), then DIPEA (2.0 mL, 11.5 mmol) was added, and the mixture was stirred at 24° C. for 6 h. Concentrate under reduced pressure to remove the solvent, and separate by silica gel column chromatography. The eluent is dichloromethane: methanol (v / v=50:1) to obtain a white solid 5-((2'-tert-butyloxycarbonylamino)- L-phenylpropionyl)amino-4-oxopentanoic acid methyl ester is 1.79 g, the yield is 83.2%. 1 H NMR(400MHz, CDCl 3 )δppm: 7.19 (s, 5H), 6.73 (s, 1H), 6.24 (s, 1H), 5.38 (t, J = 7.1 Hz, 1H), 4.12 (d, J = 12.5 Hz, 1H), 3.63 ( s, 3H), 3.60–3.47 (m, 2H), 3.11 (dd, J = 12.4, 7.0 Hz, 1H), 2.97 (dd, J = 12.3, 4.7, 1.4 Hz, 1H), 2.60 (dd, J = 12.3,4.7,1.4Hz,1H), 2.38(td,J=12.3,1.4...
Embodiment 2
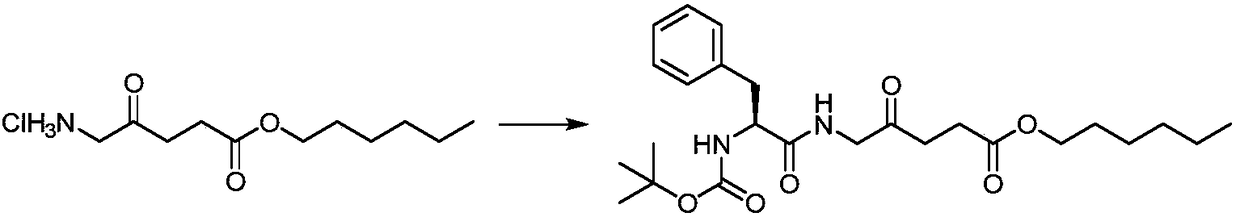
[0027] Preparation of 5-((2'-tert-butyloxycarbonylamino)-L-phenylpropionyl)amino-4-oxopentanoic acid n-hexyl ester:
[0028]
[0029] In a 50 mL round-bottom flask, mix 5-aminolevulinic acid n-hexyl ester hydrochloride (1.0g, 3.97mmol), Boc-L-phenylalanine (1.0g, 3.97mmol), HBTU (1.7g, 4.49 mmol) was dissolved in DMF (10 mL), DIPEA (1.5 mL, 8.6 mmol) was added, and the mixture was stirred at 24° C. for 6 h. Concentrate under reduced pressure to remove the solvent, and separate by silica gel column chromatography. The eluent is dichloromethane: methanol (v / v=100:1) to obtain a white solid 5-((2'-tert-butyloxycarbonylamino)- L-phenylpropionyl)amino-4-oxopentanoic acid n-hexyl ester 1.47g, the yield is 80.3%. 1 H NMR(400MHz, CDCl 3 )δppm: 7.19 (s, 5H), 6.73 (s, 1H), 6.24 (s, 1H), 5.33 (d, J = 12.5 Hz, 1H), 5.22 (t, J = 7.1 Hz, 1H), 3.97 ( dtd, J = 41.9, 12.2, 3.4 Hz, 2H), 3.71–3.59 (m, 2H), 3.09 (ddd, J = 24.4, 12.2, 6.8 Hz, 2H), 2.95 (dd, J = 12.4, 7.0 Hz, 1H), 2.79 (ddd, J = 40....
Embodiment 3
[0031] Preparation of 5-((2'-tert-butyloxycarbonylamino)-L-phenylpropionyl)amino-4-oxopentanoic acid benzyl ester:
[0032]
[0033] In a 50mL round-bottom flask, mix 5-aminolevulinic acid benzyl ester hydrochloride (1.0g, 3.88mmol), Boc-L-phenylalanine (1.0g, 3.88mmol), HBTU (1.7g, 4.49 mmol) was dissolved in DMF (10 mL), then DIPEA (2.0 mL, 11.5 mmol) was added, and the mixture was stirred at 35° C. for 6 h. Concentrate under reduced pressure to remove the solvent, and separate by silica gel column chromatography. The eluent is dichloromethane: methanol (v / v=100:1) to obtain a white solid 5-((2'-tert-butyloxycarbonylamino)- L-phenylpropionyl)amino-4-oxopentanoic acid benzyl ester 1.82g, the yield is 75.6%; 1 H NMR(400MHz, CDCl 3 )δppm: 8.31(d,J=5.7Hz,1H),7.46-7.14(m,10H),7.04(d,J=8.6Hz,1H),5.08(s,2H), 4.20(s,1H), 3.97(dd,J=12.9,5.4Hz,2H),3.04–2.95(m,1H),2.76(d,J=10.8Hz,1H),2.72(d,J=7.9Hz,2H),2.56(d ,J=6.7Hz,2H),1.29(s,9H).ESI-MS m / z:469.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com