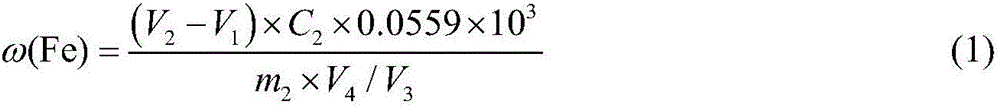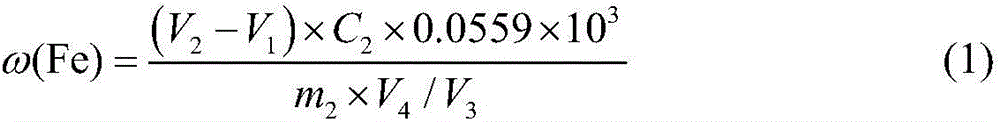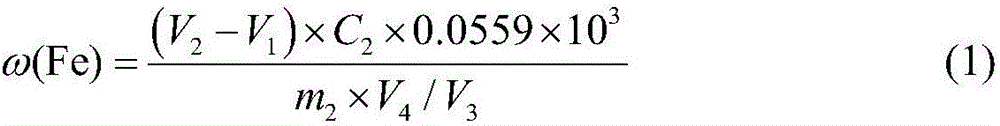Test method for chelating capability of chelating acid to metallic iron ions
A technology of chelating acid and chelating iron, which is applied in the direction of chemical analysis by titration method, can solve the problems of difficult test operation, many iron ion stabilizers, and difficult test operation results, and achieve good repeatability and easy popularization and use , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Reagent preparation:
[0050] 0.1mol / L ferric ammonium sulfate dodecahydrate solution: accurately weigh ammonium ferric sulfate dodecahydrate (NH 4 Fe(SO 4 ) 2 12H 2 O) 24.1g in a beaker, dissolved in distilled water and fixedly dissolved in a 500mL volumetric flask, shake well for later use.
[0051] 0.05mol / L EDTA standard solution: Accurately weigh 19g of ethylenediaminetetraacetic acid disodium salt, heat it, dissolve it in distilled water, and then dissolve it in a 1000mL volumetric flask, cool it, and shake it well for later use.
[0052] 2% sulfosalicylic acid solution: weigh 2.0g sulfosalicylic acid in a narrow-mouth glass bottle, add 98ml distilled water, shake well for later use.
[0053] The above reagents were all analytically pure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com