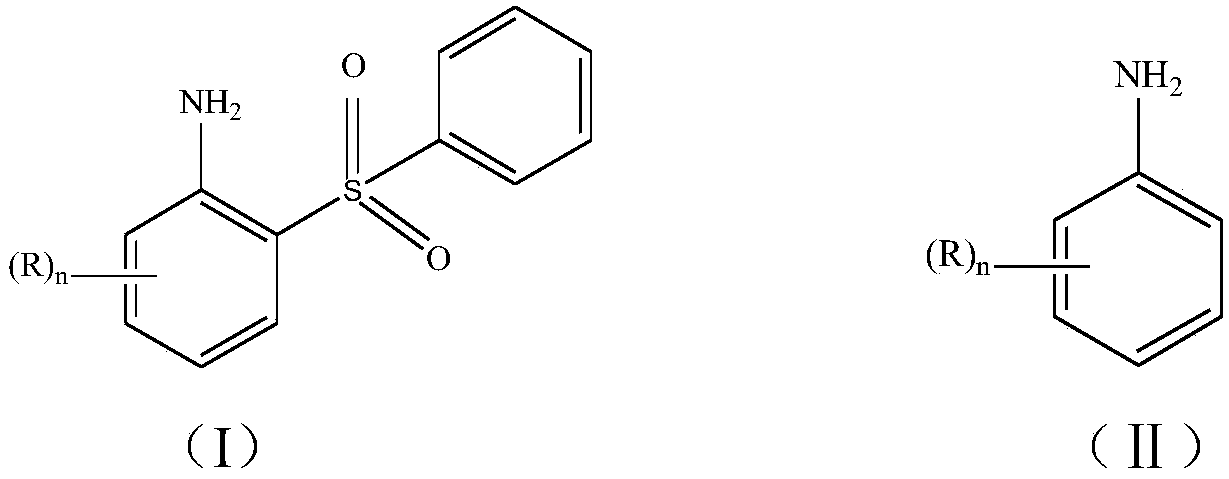
A method for preparing arylsulfonic acid compounds based on c-h activation of arylamines
An arylsulfonic acid and compound technology, which is applied in the field of copper-catalyzed arylamines to prepare arylsulfonic acid compounds, can solve the problems of long reaction time, increased research cost and difficulty, harsh reaction conditions and potential safety hazards, and achieves simple operation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
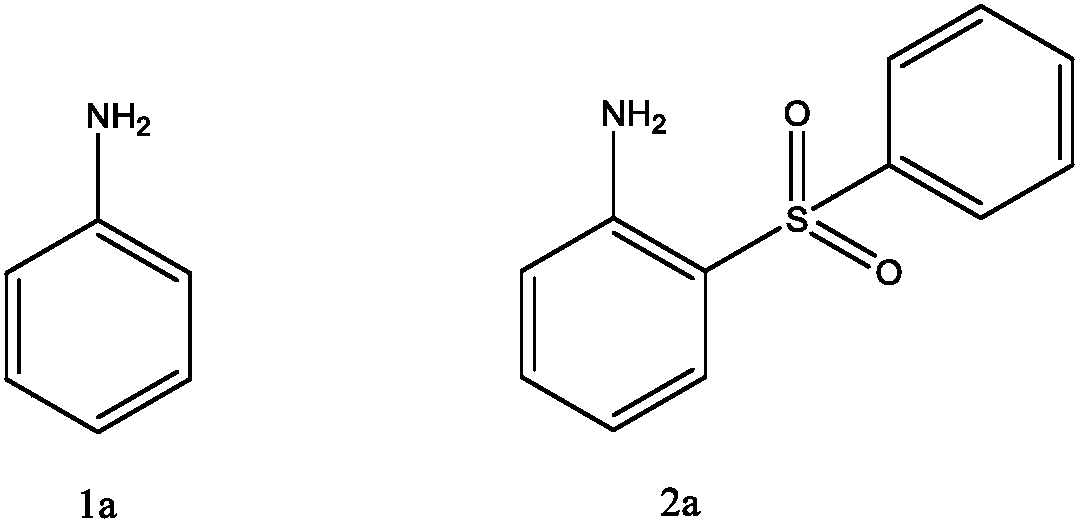
[0022] 2-(Benzenesulfonic acid)aniline
[0023]
[0024] Add 0.5mmol of aniline (1a) into 4mL of water, add 0.125mmol of copper acetate, 1.0mmol of sodium benzenesulfinate, and 1.0mmol of hydrogen peroxide, and react at room temperature for 2 hours. After the reaction, add saturated NaCl to the reaction solution The aqueous solution was extracted with ethyl acetate, and the organic layer was dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness at 60°C under reduced pressure to obtain the crude compound represented by the formula (2a). The crude compound represented by the formula (2a) was subjected to silica gel column chromatography, and a solution with a volume ratio of ethyl acetate and petroleum ether of 1:2 was used as the mobile phase, and the eluent with an Rf value of 0.3-0.5 was tracked and collected by TLC. The obtained eluent was desolventized under reduced pressure and dried to obtain 55 mg of the pure compound represented by formula (2a),...
Embodiment 2
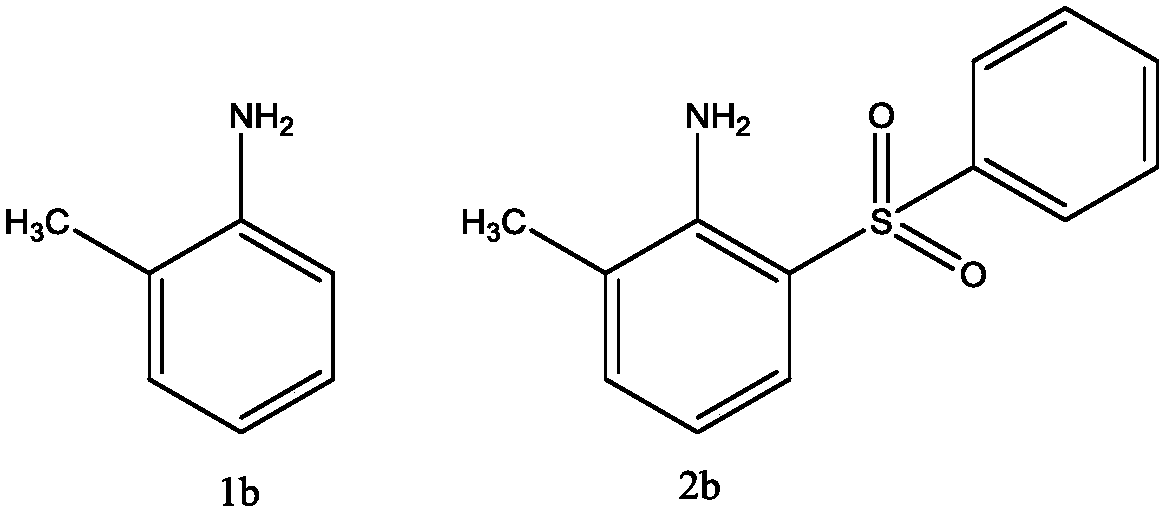
[0027] 2-Methyl-6-(benzenesulfonic acid)aniline
[0028]
[0029] Add 0.5mmol of o-toluidine (1b) into 4mL of water, add 0.125mmol of copper acetate, 1.0mmol of sodium benzene sulfinate, and 1.0mmol of hydrogen peroxide, and react at room temperature for 2 hours. After the reaction, add Saturated NaCl aqueous solution was extracted with ethyl acetate, the organic layer was dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness at 60°C under reduced pressure to obtain the crude compound represented by the formula 2b. The crude compound shown in formula 2b was subjected to silica gel column chromatography, with a solution of ethyl acetate and petroleum ether at a volume ratio of 1:2 as the mobile phase, and the eluent with an Rf value of 0.3-0.5 was tracked and collected by TLC, and the obtained The solvent was removed from the eluent under reduced pressure and dried to obtain 51 mg of the pure compound represented by formula 2b, with a yield of 41%.
[...
Embodiment 3
[0032] 4-Methyl-2-(benzenesulfonic acid)aniline
[0033]
[0034] Add 0.5mmol p-toluidine (1c) to 4mL of water, add 0.125mmol copper acetate, 1.0mmol sodium benzene sulfinate, 1.0mmol hydrogen peroxide, and react at room temperature for 2 hours. After the reaction, add Saturated NaCl aqueous solution was extracted with ethyl acetate, the organic layer was dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness at 60°C under reduced pressure to obtain the crude compound represented by the formula (2c). The crude compound represented by formula (2c) was subjected to silica gel column chromatography, using a solution with a volume ratio of ethyl acetate and petroleum ether of 1:2 as the mobile phase, and followed by TLC to collect the eluent with an Rf value of 0.3-0.5, and collected The obtained eluent was desolventized under reduced pressure and dried to obtain 49 mg of the pure compound represented by formula (2c), with a yield of 40%.
[0035] 1 H NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com