Method for preparing active peptide metal chelate by enzymatic hydrolysis of laver
A metal chelate and active peptide technology, applied in the field of laver enzymatic hydrolysis to prepare active peptide metal chelate, can solve problems such as low utilization rate, and achieve product safety, improved glycosidase inhibitory activity, and good gastrointestinal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Enzymolysis and preparation of laver α-glucosidase inhibitory active peptide
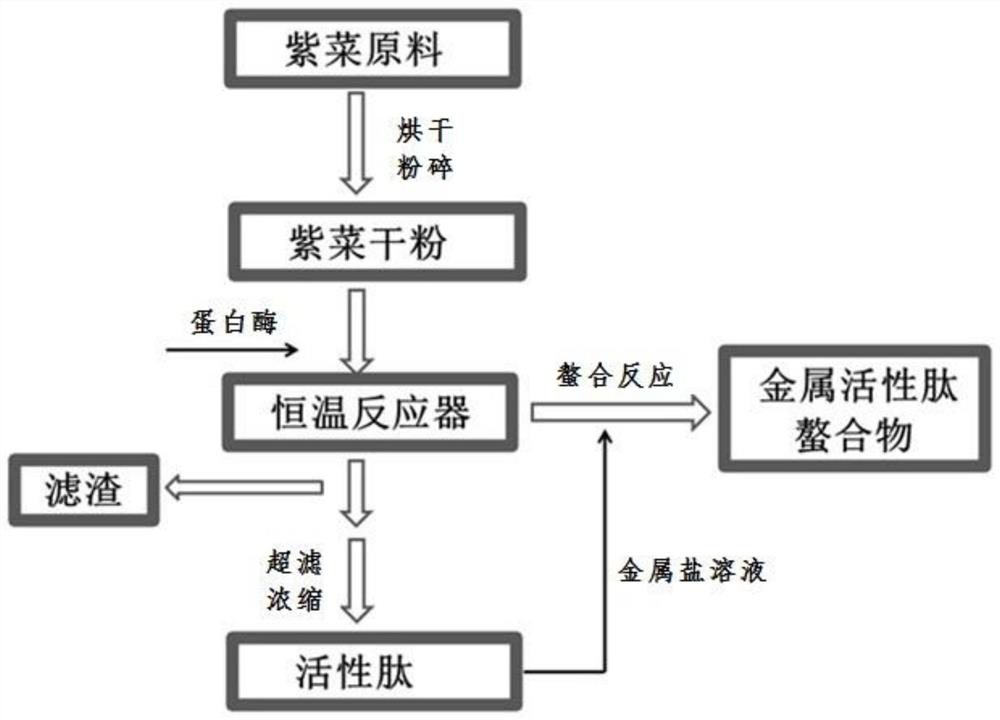
[0047] Take Porphyra zebra as raw material, dry, pulverize, sieve, take 40-mesh laver protein dry powder and add it to 20mmol / L, pH8.5 NaHCO 3 -NaH 2 CO 3 In the buffer solution, the mass ratio of the laver protein dry powder to the buffer solution is 1:33g / mL. Then add alkaline protease E / S=8×10 4 U / g, neutral protease 8×10 4 U / g dry substrate, aminopeptidase 28U / g, hydrolyze at 50°C for 6h. Alkaline protease was purchased from Pangbo Company, neutral protease was purchased from Su Kehan Company, and its model was SUKAPro NE. Aminopeptidase was independently developed and prepared by the inventor. The preparation method is described in patent CN104293749A. The enzyme was inactivated in a boiling water bath, and the supernatant was obtained by centrifugation. After diluting the supernatant 1.6 times, pass the filter membranes of 10kDa ultrafiltration membrane and 1kDa nanofi...
Embodiment 2
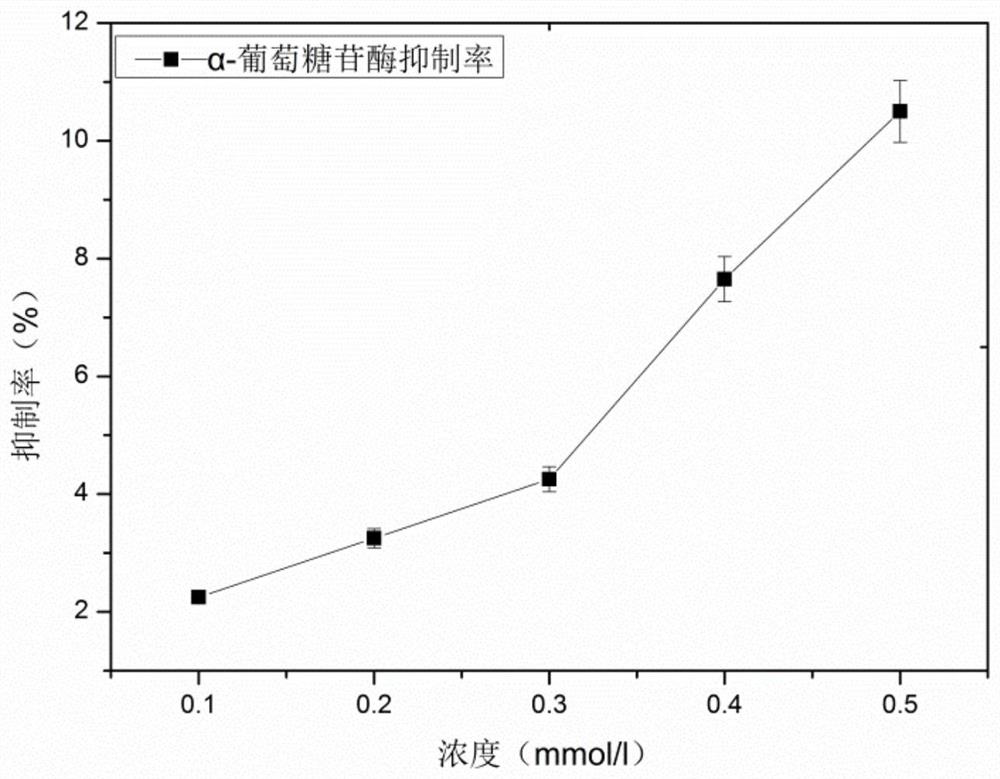
[0049] Embodiment 2 ZnCl alone 2 Effect of Solution on Inhibitory Activity of α-Glucosidase
[0050] Prepare ZnCl with a concentration of 0.1-0.5mmol / L respectively 2 solution, to measure the influence of different concentrations of metal solutions on the inhibitory rate of α-glucosidase, such as figure 2 As shown, with ZnCl 2 With the increase of solution concentration, the inhibition rate of α-glucosidase gradually increased, indicating that ZnCl alone 2 The solution has inhibitory effect on α-glucosidase.
Embodiment 3
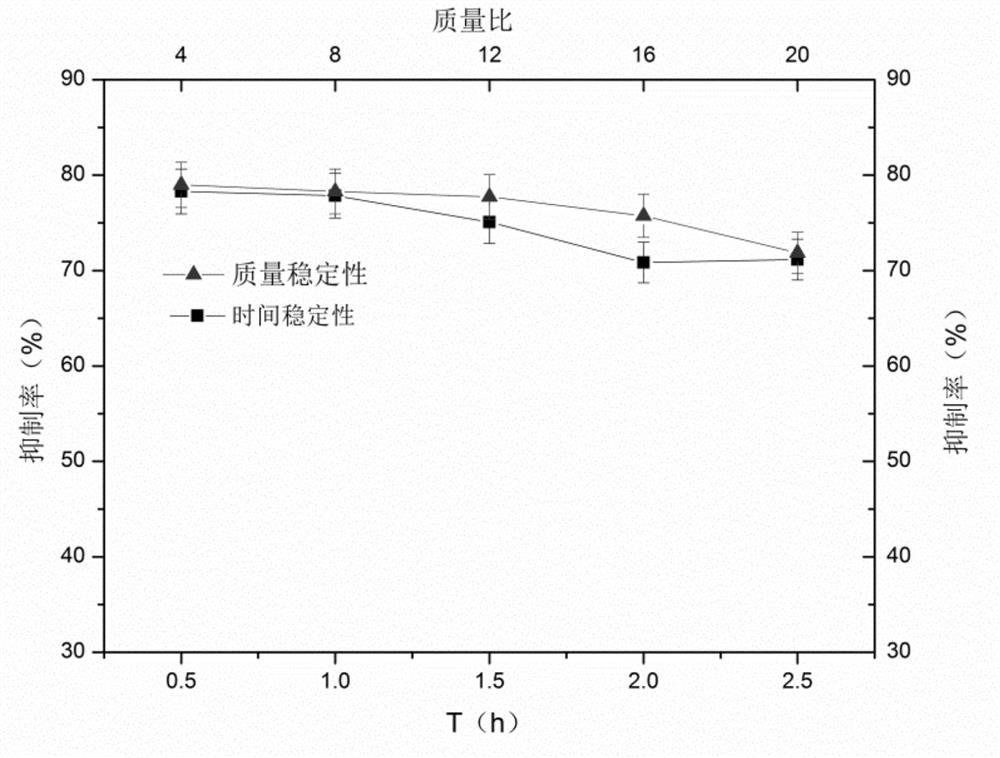
[0051] Example 3 Preparation of laver enzymatic hydrolysis product α-glucosidase inhibitory activity polypeptide zinc chelate
[0052] 0.18g laver enzymatic hydrolyzate α-glucosidase inhibitory activity polypeptide was dissolved in 30mL ZnCl concentration of 0.5mmol / L 2 solution, so that the concentration of the laver enzymatic hydrolysis product α-glucosidase inhibitory activity polypeptide in the solution is 6 mg / ml. Adjust the pH of the solution to 4.5, and then place it in a oscillating water-bath shaker at 37° C. for 1.5 h to obtain a zinc chelate of a laver enzymatic hydrolysis product α-glucosidase inhibitory active polypeptide. Its chelation degree was measured to be 25.6%. The product was made into a solution with a concentration of 1 mg / ml, and its α-glucosidase inhibition rate was measured according to the method in the above specification, and the result was 79.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Chelation degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com