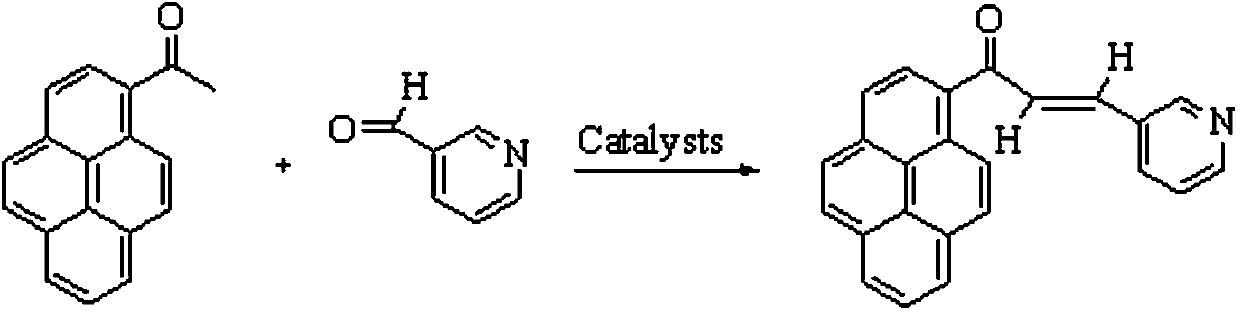
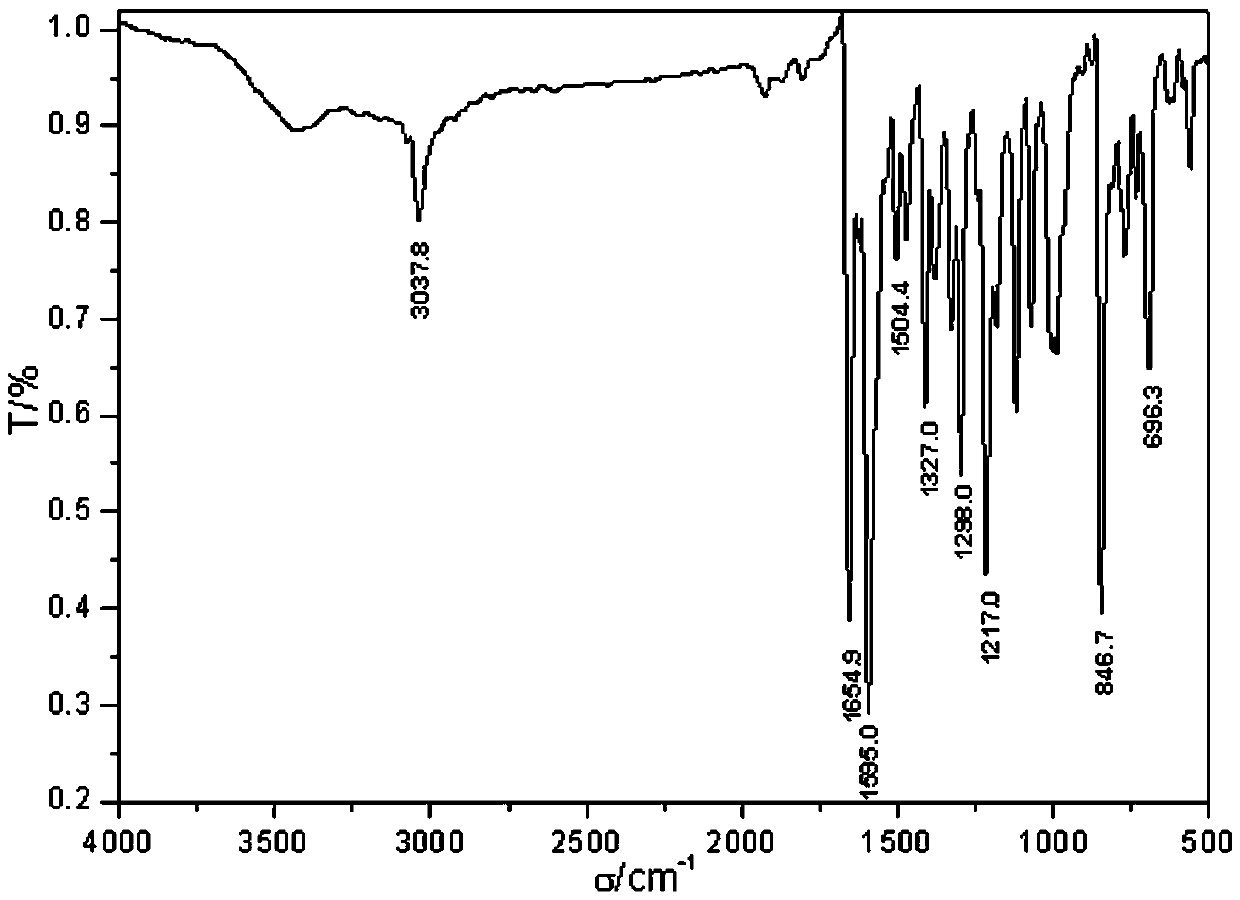
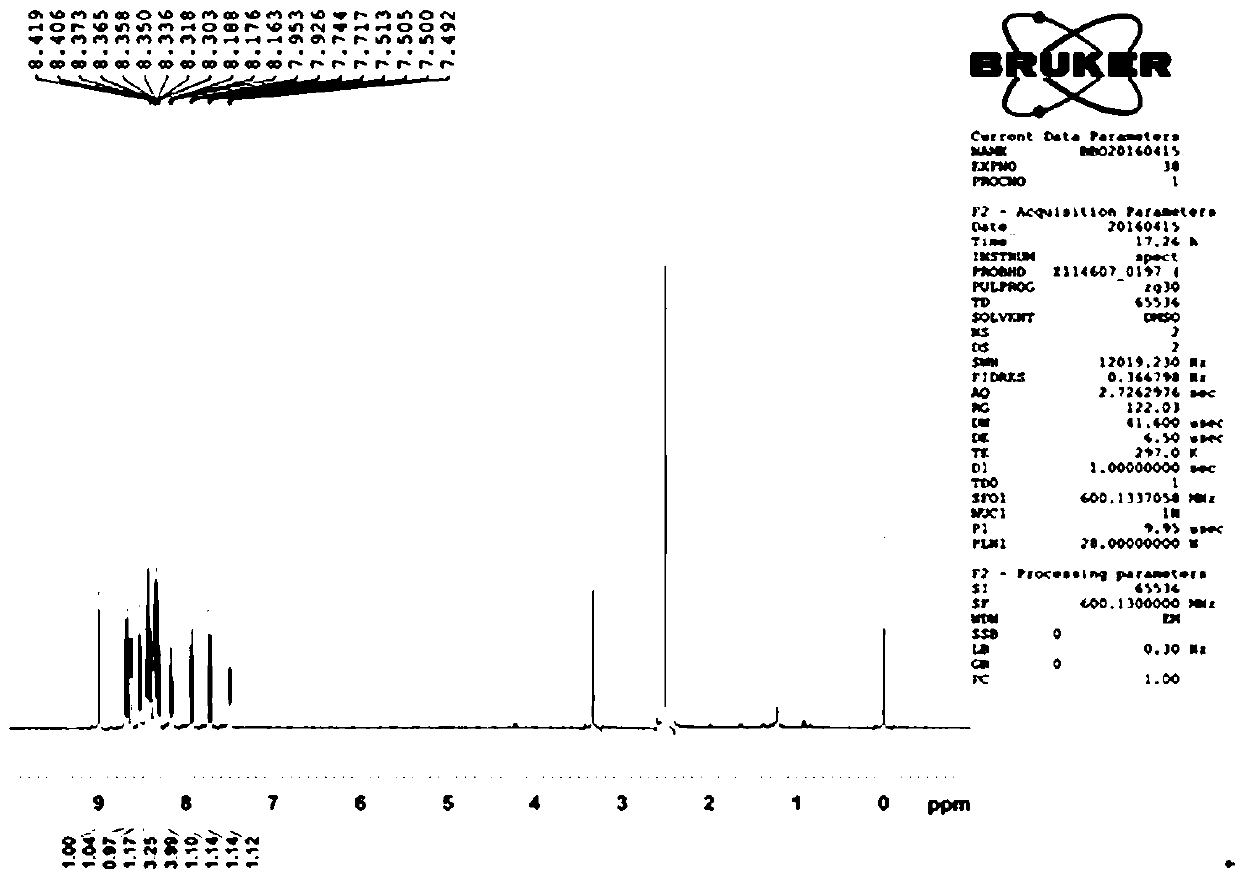
A novel pyrenylchalcone derivative and its synthesis method
A technology of pyrenylchalcone and synthesis method, which is applied in the field of novel pyrenylchalcone derivatives and their synthesis, and can solve problems such as inability to react to obtain chalcone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1, the synthetic method of described novel pyrenylchalcone derivatives, comprises the following steps:
[0061] (1) Synthesis of 1-(pyrene-1-yl)-3-(pyridin-3-yl)propenone:
[0062] In a 250 mL round bottom flask equipped with a magnetic stirrer, add 0.24 g of 1-acetylpyrene and 35 mL of absolute ethanol successively, heat and stir to dissolve them; then add 0.25 mL of 3-pyridinecarbaldehyde and catalyst sodium acetate, the amount of catalyst added 5% of the molar mass of the remaining reactants, stirred and reacted at 40°C for 4h, a yellow solid was precipitated, during which TLC tracked the reaction (developing agent was V 乙酸乙酯 : V 石油醚 =1:7), until the complete reaction of 1-acetylpyrene, stop stirring, cool, filter with suction, wash the filter cake with water and ethanol successively, filter with suction, and dry in vacuum to obtain 0.29g of the product, which is separated by column chromatography Pure product 0.27g (the eluent used for column chromato...
Embodiment 2
[0065] Embodiment 2, the synthetic method of described novel pyrenylchalcone derivatives, comprises the following steps:
[0066] (1) Synthesis of 1-(pyrene-1-yl)-3-(pyridin-3-yl)propenone:
[0067] In a 250 mL round bottom flask equipped with a magnetic stirrer, add 0.24 g of 1-acetylpyrene and 35 mL of absolute ethanol successively, heat and stir to dissolve them; then add 0.25 mL of 3-pyridinecarbaldehyde and catalyst sodium acetate, the amount of catalyst added 7% of the molar mass of the remaining reactants, stirred and reacted at 50°C for 5h, a yellow solid was precipitated, during which TLC tracked the reaction (developing agent was V 乙酸乙酯 : V 石油醚 =1:7), until the complete reaction of 1-acetylpyrene, stop stirring, cool, filter with suction, wash the filter cake with water and ethanol successively, filter with suction, and dry in vacuum to obtain 0.28g of the product, which is separated by column chromatography Pure product 0.26g (the eluent used for column chromato...
Embodiment 3
[0070] Embodiment 3, the synthetic method of described novel pyrenylchalcone derivatives, comprises the following steps:
[0071] (1) Synthesis of 1-(pyrene-1-yl)-3-(pyridin-3-yl)propenone:
[0072] In a 250 mL round bottom flask equipped with a magnetic stirrer, add 0.24 g of 1-acetylpyrene and 35 mL of absolute ethanol successively, heat and stir to dissolve them; then add 0.25 mL of 3-pyridinecarbaldehyde and catalyst sodium acetate, the amount of catalyst added 10% of the molar mass of the remaining reactants, stirred and reacted at 60°C for 6h, a yellow solid was precipitated, during which TLC tracked the reaction (developing agent was V 乙酸乙酯 : V 石油醚 =1:7), until the complete reaction of 1-acetylpyrene, stop stirring, cool, filter with suction, wash the filter cake with water and ethanol successively, filter with suction, and dry in vacuum to obtain 0.27g of the product, which is separated by column chromatography Pure product 0.25g (the eluent used for column chromat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com