Virus-like particle vaccines
A virus-like, virus-like technology, applied to viruses, vaccines, viral peptides, etc., can solve problems such as interference with antigen folding, failure to maintain antigens and antigen fragments, and insufficient maintenance of the original antigen configuration to achieve strong immunogenicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Embodiment 1: Construction of virus-like particle template
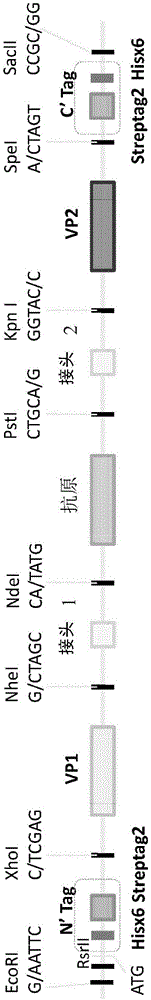
[0178] The virus-like particle template ( FIG. 1 ) was synthesized in plasmid pUC57 with GenScript, and the sequence was optimized for codons suitable for yeast. The fragment of the virus-like particle template was subcloned into the yeast expression plasmid vector pPICZ A using EcoRI (5'-end) and SacII (3'-end) cleavage, and the expression was regulated by the methanol-inducible AOX1 promoter. The sequence encoding the N-terminal viral structural protein (VP1) was cloned into the template using the XhoI+Nhel paired restriction sites at the 5' and 3' ends. The sequence encoding the N-terminal linker (linker 1 ) was cloned into the template using the NheI+NdeI paired restriction sites at the 5' and 3' ends. The sequence encoding the antigen was cloned into the template using the NdeI+PstI paired restriction sites at the 5' and 3' ends. The sequence encoding the C-terminal linker (linker 2) was cloned into the...
Embodiment 1A
[0186] Example 1A: Construction of S-VP1-S
[0187] The coding region of S-VP1-S was synthesized in plasmid pUC57 with GenScript (see Figure 9 ), and the sequence was optimized for codons suitable for the E.coli host. The fragment of the virus-like particle template was subcloned into the plastid vector pCRT7NT (Invitrogen) by PCR, and the expression was regulated by the IPTG-induced T7 promoter. This technique was used to produce the recombinant proteins of Examples 1-109 in the table above.
Embodiment 2
[0188] Example 2: Yeast Transformation
[0189] Recombinant plastid DNA was linearized with PmeI restriction enzyme (NEB Company), and (Macherey-Nagel) clean up the recombinant plastids for subsequent transformations. By LiCl method and according to EasySelect TM According to the operation manual of the Pichia Expression Kit (Invitrogen), 5-10 μg of linearized plastid DNA was transformed into the Pichia host strain GS115. The transformant was inoculated on YPDS plate medium containing 50 μg / ml bleomycin (Invivogen Company) (1% (w / v) yeast extract, 2% (w / v) tryptone, 2% (w / v) v) Glucose and 1.5% (w / v) agar). Colonies resistant to bleomycin were screened and the insert was confirmed by colony PCR using the following primers: 5'AOX1 primer: 5'-GACTGGTTCCAATTGACAAGC-3'(SEQ ID NO:28); 3'AOX1 primer: 5'-GCAAATGGCATTCTGACATCC-3' (SEQ ID NO: 29).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com