Application of ceredin
A technology of shertenin and influenza virus, which is applied in the field of medicine and can solve problems such as reports on the application of shertenin to influenza virus diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
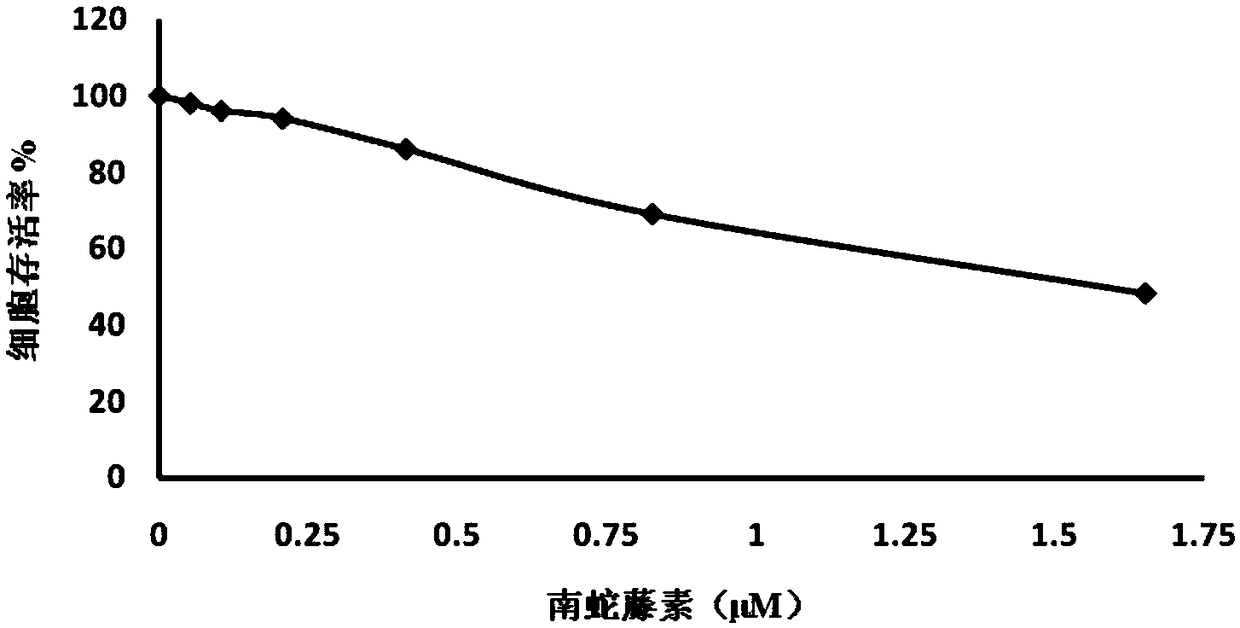
[0040] The cytotoxicity of embodiment 1 cerelin
[0041] Method steps: MDCK cells are seeded in a 96-well cell culture plate, and the drug-containing medium is added after the cells adhere to the wall. After adding the drug and culturing, observe the cytopathic effect (CPE) caused by the drug under a light microscope, add Incubate at 37°C for 2h, detect fluorescence In the case of reduction, the excitation light is 540nm, and the emission light is 595nm.
[0042] Cell activity (%)=(sample well-blank control) / (cell control-blank control)*100%
[0043] Table 1 cerelin cytotoxicity test data
[0044]
[0045] The results are shown in Table 1, figure 1 It is the toxicity of ceheriferin to MDCK dog kidney cells in Example 1 of the present invention. Depend on figure 1 It can be seen that cerelin has no cytotoxicity to MDCK cells at 0.41 μM.
Embodiment 2
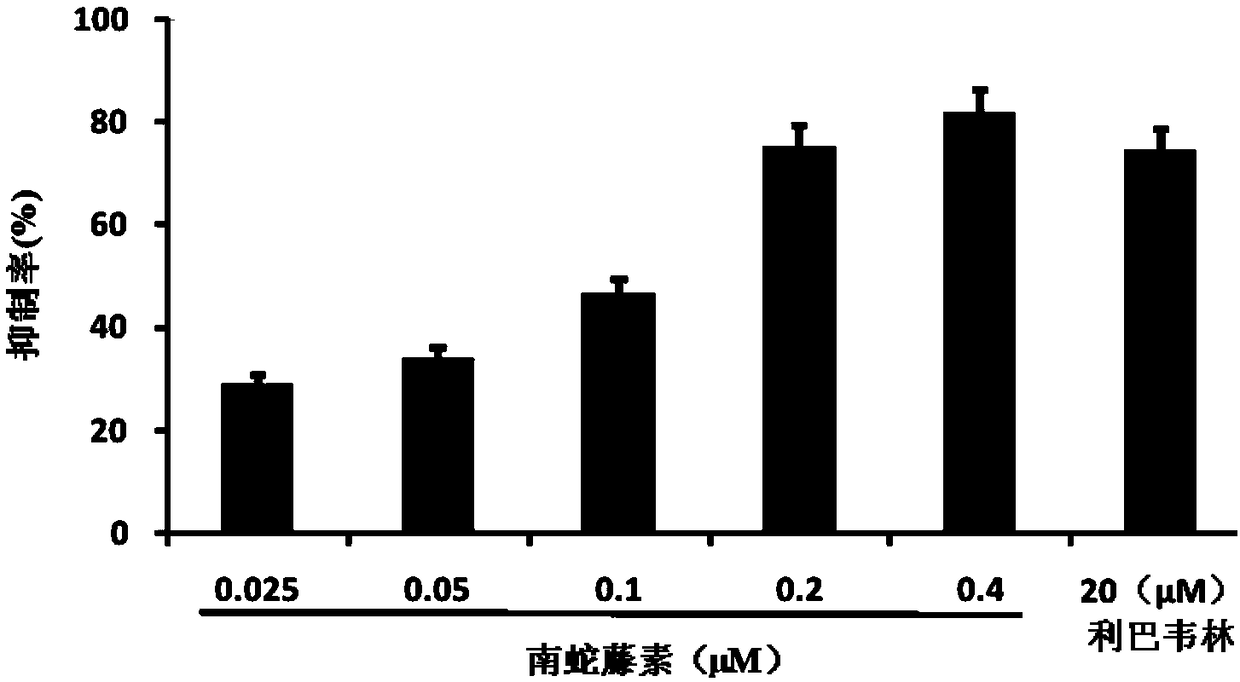
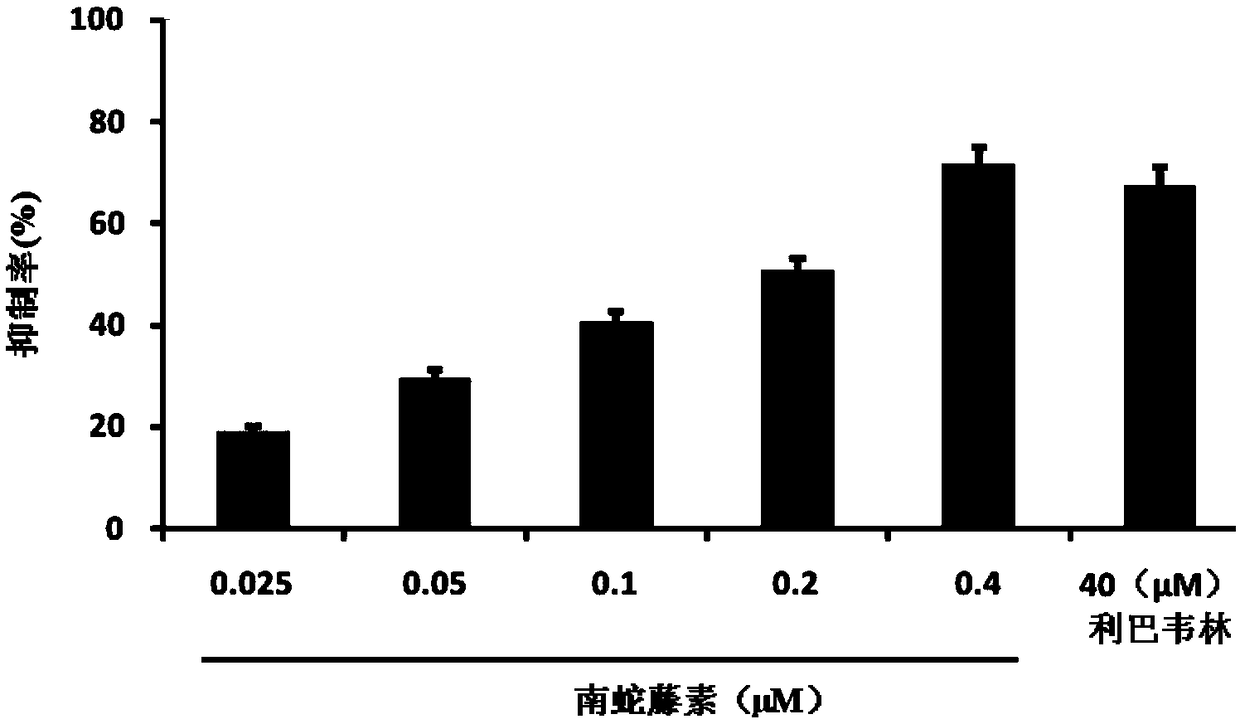
[0046] The inhibitory activity of embodiment 2 cerelin to influenza A virus
[0047] Method steps: MDCK cells were inoculated in a 96-well cell culture plate, cultured overnight at 37°C and then used for later use. After MDCK cells were washed twice with PBS, influenza virus liquid and drugs with gradient dilution concentrations were added at the same time. After cultured in a cell incubator at 37°C for 24 hours, the cytopathic changes (CPE) were observed under a microscope; the supernatant of the culture medium was taken to detect neuraminidase activity.
[0048] In the experiment, blank control wells (normal cells), virus control wells (no drug added after virus infection), and positive drug control wells (ribavirin added after infection) were set.
[0049] Inhibition rate (%)=100-(sample well-blank control) / (virus control-blank control)*100%
[0050] Table 2 The results of the inhibitory activity of ceheriferin on influenza virus strain H1N1
[0051]
[0052] Table 3 ...
Embodiment 3
[0057] Therapeutic effect of embodiment 3 shertenin gavage (oral) administration to mouse infection caused by influenza A virus
[0058] First, a preliminary experiment was performed to determine the LD50 of influenza virus A / PuertoRico / 8 / 1934 (H1N1). 80 mice were randomly divided into 4 groups, 20 in each group. They were: (1) normal control group: intragastric administration of distilled water, (2) model group: intragastric administration of distilled water, (3) positive group: intragastric administration of oseltamivir aqueous solution (20mg / kg), (4) South Shertenin group: shertenin aqueous solution (20 mg / kg) was given by intragastric administration. 4 hours before virus inoculation and 5 consecutive days after virus inoculation, once a day. After ether anesthesia, 10LD 50 Dosage Nasal inoculation of influenza virus 50 μL. Take 10 mice in each group for continuous observation for 14 days, and record the death situation of the mice (calculate the death rate and average s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com