Beta2-receptorexcitant and preparation method and application thereof
A solvate, C1-C4 technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
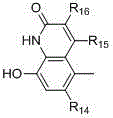
[0067] Synthesis of 8-Hydroxy-5-(2-Hydroxy-1-isopropylaminoethyl)-(1H)-quinolin-2-one Hydrochloride
[0068] a. Synthesis of 8-benzyloxy-5-(1-bromo-2-hydroxyethyl)-(1H)-quinolin-2-one
[0069] 40g (0.108mol) 8-benzyloxy-5-bromoacetyl-(1H)-quinolin-2-one (according to WO2006023457A 1 The preparation) was dissolved in a mixed solution of 800mL methanol and 400mL dichloromethane, and 1.64g (0.0429mol) sodium borohydride was added in batches under an ice bath. After the reaction was completed, 2N hydrochloric acid was added to make the solution acidic, and the solvent was evaporated to dryness. Add water and stir thoroughly, and obtain 38.0 g of a yellow solid after suction filtration and drying, with a yield of 94.5%.
[0070] b. Synthesis of 8-benzyloxy-5-oxiranyl-(1H)-quinolin-2-one
[0071] Dissolve 38.0g (0.102mol) of 8-benzyloxy-5-(1-bromo-2-hydroxyethyl)-(1H)-quinolin-2-one in a mixed solution of 760mL methanol and 380mL dichloromethane , drop 5.71g (0.102mol) of potassi...
Embodiment 2
[0078] According to the method similar to Example 1, 8-hydroxyl-5-(2-hydroxyl-1-tert-butylamine was prepared from 8-benzyloxy-5-oxiranyl-(1H)-quinolin-2-one as raw material Ethyl)-(1H)-quinolin-2-one hydrochloride.
[0079] 1 H-NMR (300MHz, DMSO-d 6 ,ppm):1.23(9H,s),3.79-3.85(2H,m),4.87(1H,m),5.50(1H,s),6.58-6.61(1H,d,J=9.90Hz),7.03- 7.06 (1H, d, J = 8.28Hz), 7.43-7.46 (1H, d, J = 8.28Hz), 8.31-8.35 (1H, d, J = 9.99Hz), 8.54 (1H, s), 9.06 (1H ,s), 10.53(1H,s), 10.70(1H,s).
Embodiment 3
[0081] According to the method similar to Example 1, 8-hydroxyl-5-(2-hydroxyl-1-n- Propylaminoethyl)-(1H)-quinolin-2-one hydrochloride.
[0082] 1 H-NMR (300MHz, DMSO-d 6 ,ppm):0.79-0.84(3H,t),1.59-1.73(2H,m),2.67(1H,m),2.84(1H,m),3.75-3.90(2H,m),4.86(1H,m ),5.53(1H,s),6.57-6.60(1H,d,J=9.90Hz),7.05-7.08(1H,d,J=8.28Hz),7.38-7.40(1H,d,J=8.28Hz) , 8.18-8.22 (1H, d, J = 10.08Hz), 9.08 (1H, s), 9.36 (1H, s), 10.54 (1H, s), 10.74 (1H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com