Preparation method of epalrestat dispersible tablet
A technology of epalrestat and dispersible tablets, which is applied in the field of preparation of epalrestat dispersible tablets, can solve problems such as easy energy consumption, unfavorable large-scale production, and increased costs, so as to reduce process operation steps, reduce input costs, and save energy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The selection test of embodiment 1 epalrestat dissolving solvent
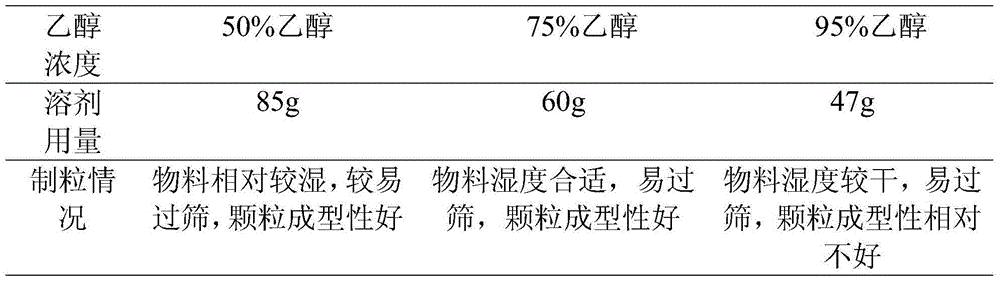
[0025] Take about 50 mg of the raw materials and put them in a beaker, gradually add ethanol solutions of different concentrations (50%, 75%, 95%), observe while shaking until the solution is clear, and weigh the amount of solvent added. According to the amount of different ethanol concentrations, 150 mg of mixed auxiliary materials were used to make soft materials, and the humidity of the soft materials was observed, and sieved with a 20-mesh sieve. The results are shown in Table 1.
[0026] Table 1 The selection and investigation results of the main ingredient dissolving solvent
[0027]
[0028] Results: The above-mentioned ethanol solutions with different concentrations can dissolve 50mg of raw materials well under a certain amount, have a good wetting effect on 150mg, and have good formability on the material particles. It is confirmed that the granulation solvent of this product uses 50-95% etha...
experiment example 2
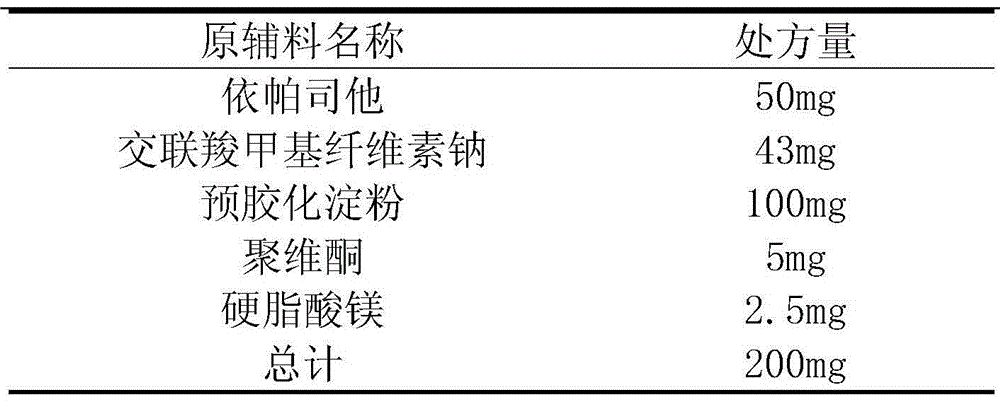
[0030] The prescription of epalrestat tablets consists of the ingredients shown in Table 2.
[0031] Table 2 Prescription of Epalrestat Tablets
[0032]
[0033] The preparation method of above-mentioned epalrestat dispersible tablet is as follows:
[0034] Preparation Process:
[0035] 1. Dissolve the prescribed amount of epalrestat and povidone ultrasonically in about 60g of 75% ethanol solution at the same time, as the adhesive containing the main drug, the adhesive is colorless and transparent, without lumps and bubbles .
[0036] 2. Take the prescribed amount of sodium carboxymethyl starch and pregelatinized starch and mix them through a 100-mesh sieve for 3 times.
[0037] 3. Add the binder containing the main ingredient to the mixture in step 2, prepare the soft material, granulate with a 20-mesh sieve, and dry in an oven at 60°C (moisture content 1-3%).
[0038] 4. The granules are passed through a 24-mesh sieve for granulation, and magnesium stearate is added a...
experiment example 3
[0041] The prescription of epalrestat tablets consists of the ingredients shown in Table 3.
[0042] Table 3 Prescription of Epalrestat Tablets
[0043]
[0044] The preparation method of above-mentioned epalrestat dispersible tablet is as follows:
[0045] Preparation Process:
[0046] 1. Dissolve the prescribed amount of epalrestat and hydroxypropyl methylcellulose in about 85g of
[0047] In 50% ethanol solution, as the adhesive containing the main drug, the adhesive is colorless and transparent without lumps and bubbles.
[0048] 2. Take the prescribed amount of microcrystalline cellulose and cross-linked polyvinylpyrrolidone and mix them through a 100-mesh sieve for 3 times.
[0049] 3. Add the binder containing the main ingredient to the mixture in step 2, prepare the soft material and granulate it with a 20-mesh sieve, and dry it in an oven at 40°C (moisture content 1-3%).
[0050] 4. The granules are passed through a 24-mesh sieve for granulation, and magnesium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com