Preparation method of ivermectin
A technology for ivermectin and abamectin, applied in the field of biological drug preparation, can solve the problems of many crystallization times, high cost, low yield and the like, and achieve the effects of high yield, low production cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Prepare the crude product of ivermectin: add 500ml of toluene in the hydrogenation kettle, add 150g of high-quality abamectin from the feed port of the hydrogenation kettle, add 1.3g of catalyst under stirring with nitrogen gas, close the feed port of the autoclave, Replace the air in the hydrogenation tank with nitrogen, then replace the nitrogen in the hydrogenation tank with hydrogen, then heat up to 68°C, open the hydrogen valve until the pressure of the reactor rises to 0.5MPa to carry out the hydrogenation reaction, and take a sample for 1 hour. Until the central control is qualified, the reaction time is 1 hour and 45 minutes; put the above-mentioned feed liquid into the catalyst removal kettle, add 2 g of thiourea, keep the water bath at 50-60 ° C, stir for 2 hours under the protection of nitrogen, then cool down to 30 ° C, pump Filter, obtain the crude product 148g of ivermectin;
[0030] 2) Dissolve it with 650ml of ethanol, heat at 70°C, stir until complet...
Embodiment 2
[0038] 1) Prepare the crude product of ivermectin: add 500ml of toluene in the hydrogenation kettle, add 150g of high-quality abamectin from the feed port of the hydrogenation kettle, add 1.3g of catalyst under stirring with nitrogen gas, close the feed port of the autoclave, Replace the air in the hydrogenation tank with nitrogen, then replace the nitrogen in the hydrogenation tank with hydrogen, then heat up to 68°C, open the hydrogen valve until the pressure of the reactor rises to 0.5MPa to carry out the hydrogenation reaction, and take a sample for 1 hour. Until the central control is qualified, the reaction time is 1 hour and 45 minutes; put the above-mentioned feed liquid into the catalyst removal kettle, add 2 g of thiourea, keep the water bath at 50-60 ° C, stir for 2 hours under the protection of nitrogen, then cool down to 30 ° C, pump Filter, obtain the crude product 148g of ivermectin;
[0039] 2) Dissolve it with 650ml of ethanol, heat at 75°C, stir until complet...
Embodiment 3
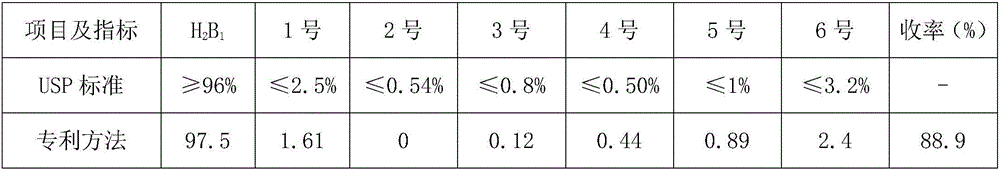
[0048]What this embodiment studies is the influence of cooling control conditions on the crystallization of ivermectin. Other test conditions are consistent with those in Example 1. The changed test conditions are that the cooling process of the solution adopts a water bath cooling method. After dropping to 35°C, Replace it with cold brine, and cool down to 15°C to end. The test data are shown in Table 3. It can be seen from the table below that the different cooling methods in the crystallization process affect the selectivity of crystallization. Judging from the impurities of No. 1 to No. 6 in the product, the quality of ivermectin crystals obtained by gradient cooling method will be better than that of Crystallization quality obtained by water bath cooling method. Therefore, what the preparation method of the present invention preferably adopts is gradient cooling method.
[0049] table 3
[0050] Items and Indicators
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com