A fluorescent probe compound and its preparation method and use
A technology of derivatives and hydrazine hydrate, which is applied in the field of fluorescent probes, can solve the problems of complex instruments and equipment, tediousness, cell damage, etc., and achieve the effects of consistent reaction conditions, simple synthesis routes, and simple and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: detect the preparation of the fluorescent probe of iron ion, basic synthesis process is as follows:
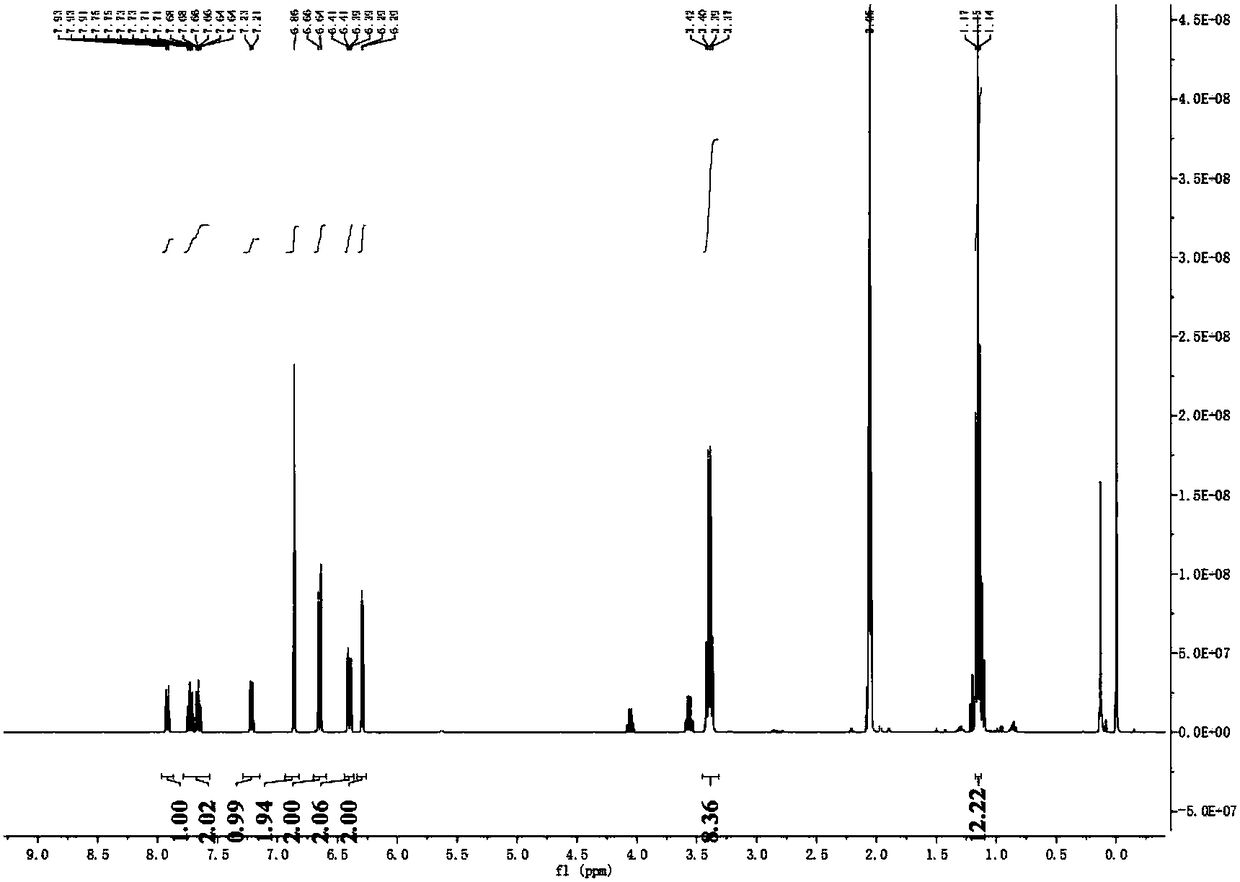
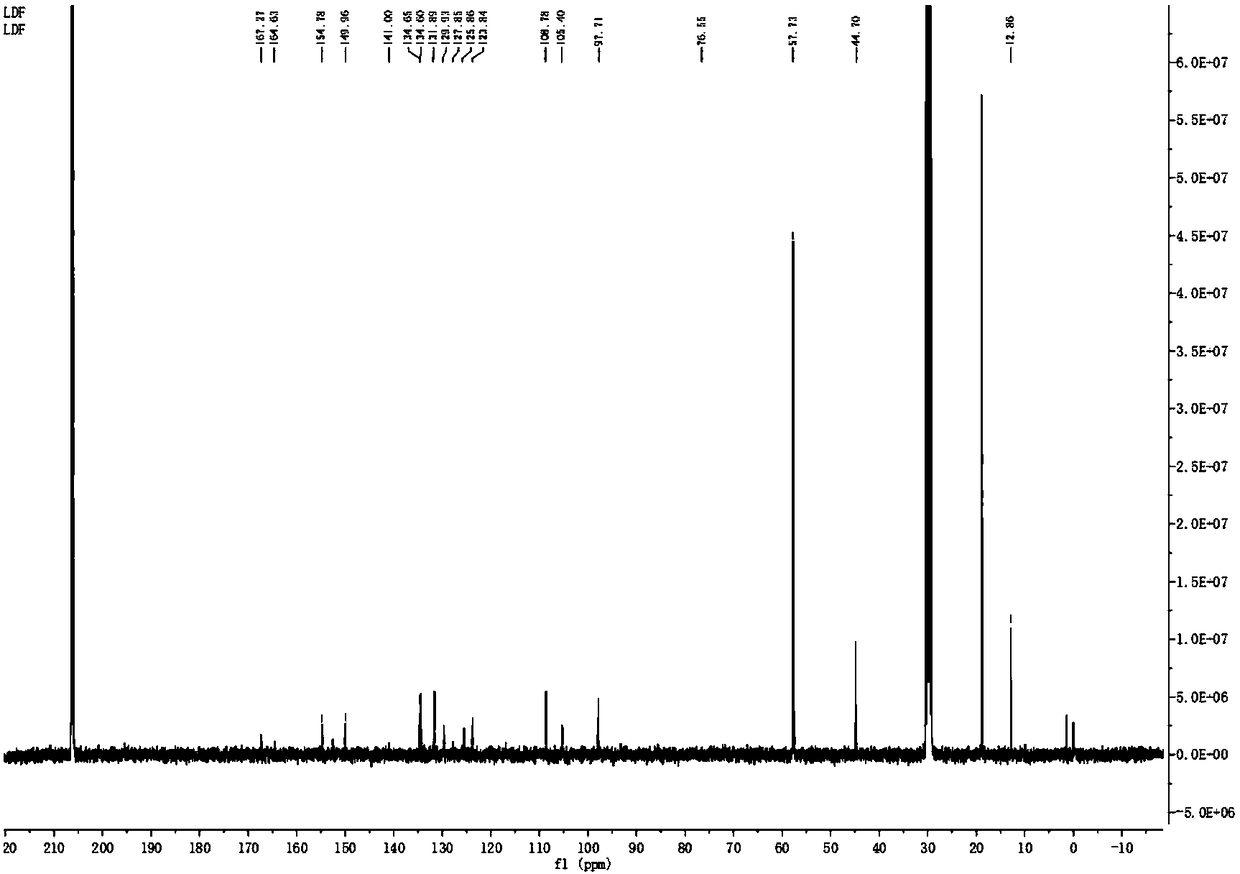
[0032] (1) Synthesis of Rhodamine Hydrazine Hydrate: Add 2 mL of ethanol dropwise to a 25 mL one-mouth bottle, add 1 mmol of Rhodamine B to it and stir to dissolve it; React for 1 hour to obtain an orange-yellow solution, and rotary evaporate to obtain a viscous solid, then add hydrochloric acid solution dropwise therein until the solid is completely dissolved, then add sodium hydroxide solution dropwise therein until the pH reaches 8, pink flocs appear, pump Filter to obtain a pink solid: 90% yield.
[0033] (2) Synthesis of probe molecules: the 0.9mmol rhodamine hydrazine hydrate obtained in step (1) was dissolved in 10mL glacial acetic acid, then 0.9mmol maleic anhydride was added thereto, reflux reaction was performed for 4 hours, and rotary evaporation, Then the yellow solid rhodamine hydrazide derivative was separated by column chromatography, and t...
Embodiment 2
[0034] Embodiment 2: detect the preparation of the fluorescent probe of iron ion and mercaptan, basic synthesis process is as follows:
[0035] 1) Synthesis of Rhodamine Hydrazine Hydrate: Add 35 mL of ethanol dropwise to a 150 mL one-mouth bottle, add 10 mmol Rhodamine B to it and stir to dissolve it; then add 30 mmol L Hydrazine Hydrate dissolved in 20 mL ethanol dropwise into Rhodamine for reflux reaction 4 hour, an orange solution was obtained, rotary evaporation obtained a viscous solid, sulfuric acid was added dropwise therein until the solid was completely dissolved, and then potassium hydroxide solution was slowly added dropwise until the pH reached 10, pink flocs appeared, suction filtered to obtain a pink solid : 90% yield.
[0036]2) Synthesis of probe molecules: 5mmol rhodamine hydrazine hydrate obtained in step (1) was dissolved in 35mL glacial acetic acid, then 15mmol maleic anhydride was added thereto, refluxed for 7 hours, rotary evaporation, column chromatogra...
Embodiment 3
[0037] Embodiment 3: the fluorescence spectrometry of probe molecule
[0038] Get 3mL concentration among the example 1 and be the probe ethanol water mixed solution of 10 μ mol / L in the quartz cuvette, then add 3 μ L concentration respectively and be the various metal ions of 10 mmol / L (Na + , K + , Ag + ,Mg 2+ ,Cu 2+ ,Cd 2+ ,Pb 2+ ,Zn 2+ ,Mn 2+ ,Co 2+ , Ni 2+ , Fe 3+ ,Al 3+ ) solution, shake well, and measure its fluorescence emission spectrum at an excitation wavelength of 455nm (such as Figure 4 ), the results show that when no metal ions are added, there is no emission peak; when ferric ions are added, an emission peak appears at 581nm; when other metal ions are added, no emission peaks appear; change as Figure 5 As shown, the color changes from colorless to purple; under fluorescent light, the color changes as Figure 6 As shown, the color changed from colorless to pink fluorescent.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap