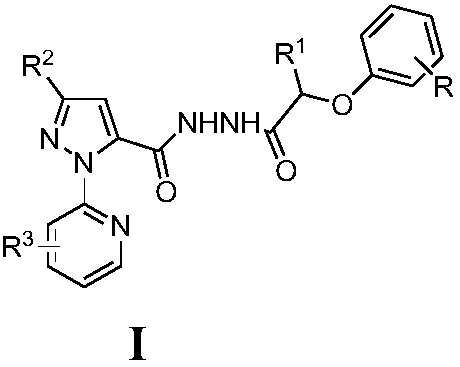
n1-(3-substituted pyrazole-5-formyl)-substituted phenoxyalkylhydrazide derivatives and their application
A technology of phenoxyalkyl hydrazide and formyl, which can be applied in application, biocide, animal repellent, etc., can solve the problem of reducing the sensitivity of pest populations, and achieve the effect of enriching species and alleviating the problem of drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
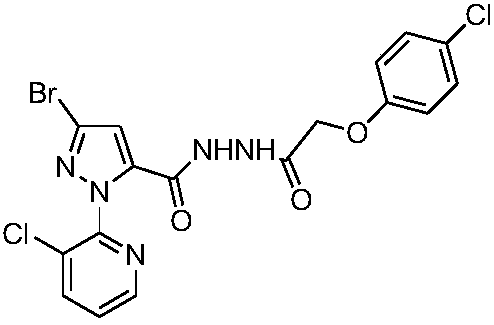
[0020] Compound I-1: 1 N-[5-(3-Bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole)-formyl]-4-chlorophenoxyacetylhydrazide
[0021]
[0022] (1) Take 0.0055mol 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid III, 3mL of thionyl chloride and 7mL of toluene and add it into a container equipped with a reflux condenser and a thermometer In a 25mL three-necked flask, heat up to 50-80°C for 4-5 hours, then distill off the remaining thionyl chloride and toluene to obtain 3-bromo-1-(3-chloropyridin-2-yl)- 1H-pyrazole-5-formyl chloride IV, directly used in the next step reaction;
[0023] (2) In a 50mL round bottom flask, add (0.005mol) intermediate 4-chlorophenoxyalkylhydrazide II, (0.003mol) K 2 CO 3 , 10 mL THF and 10 mL water. Add the intermediate 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carbonyl chloride IV dropwise under stirring at room temperature, after the addition is complete, follow the reaction by TLC, stir at room temperature for 0.5~3h, and filter with...
Embodiment 2
[0097] Compound I-11: 1 N-[5-(3-Bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole)-formyl]-4-fluorophenoxyisopropionylhydrazide
[0098]
[0099] (1) Take 0.0055mol 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid III and 5mL of thionyl chloride and add it into a 25mL three- In the flask, the temperature was raised to 50-80°C for 4-5 hours, and then excess thionyl chloride was evaporated to obtain 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole- 5-Formyl chloride IV is directly used in the next step reaction;
[0100] (2) Add (0.005 mol) intermediate 4-fluorophenoxyisopropionohydrazide II, (0.006 mol) triethylamine, 10 mL THF and 10 mL water into a 50 mL round bottom flask. Add the intermediate 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carbonyl chloride IV dropwise under stirring at room temperature, after the addition is complete, follow the reaction by TLC, stir at room temperature for 0.5~3h, and filter with suction , washed with water, recrystallized from et...
Embodiment 3
[0158] Compound I-19: 1 N-[5-(3-Trifluoromethyl-1-(pyridin-2-yl)-1H-pyrazole)-formyl]-4-chlorophenoxyacetylhydrazide
[0159]
[0160] (1) Take 0.0055mol 3-trifluoromethyl-1-(pyridin-2-yl)-1H-pyrazole-5-carboxylic acid III, 5mL of thionyl chloride, and 6mL of toluene and add them into a container equipped with a reflux condenser and a thermometer. In a 25mL three-necked flask, heat up to 50-80°C and react for 4-5 hours, then evaporate excess thionyl chloride to obtain 3-trifluoromethyl-1-(pyridin-2-yl)-1H- Pyrazole-5-carbonyl chloride IV was directly used in the next reaction.
[0161] (2) Add (0.005mol) intermediate 4-chlorophenoxyacetylhydrazide II in a 50mL round bottom flask, (0.006mol) K 2 CO 3 , 15 mL of water. Add the intermediate 3-trifluoromethyl-1-(pyridin-2-yl)-1H-pyrazole-5-carbonyl chloride IV dropwise under stirring at room temperature, after the addition is complete, follow the reaction by TLC, stir at room temperature for 0.5~3h, and filter with suction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com