Synthetic method for Velpatasvir intermediate
A synthetic method, the technology of velpatasvir, is applied in the field of synthesis of the key core structure of the new hepatitis C virus drug velpatasvir and its series of intermediates, which can solve the problem that trimethylsilylacetylene is expensive and unsuitable for scale-up Production, low boiling point, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
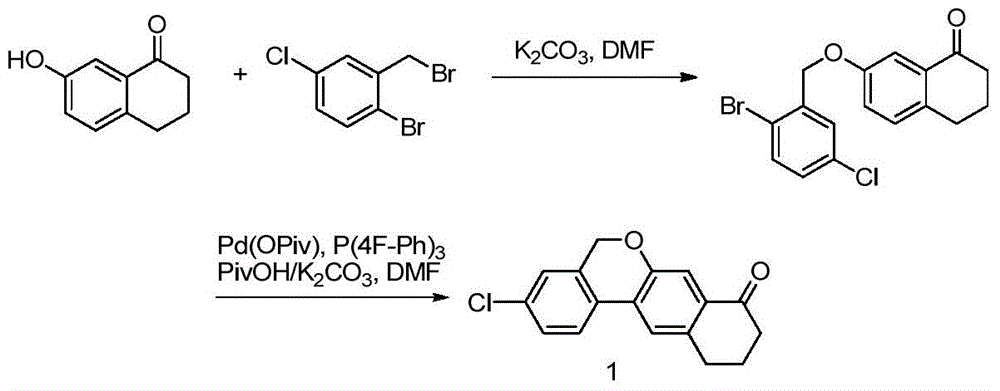
[0050]Add compound 6 (28.44g, 100mmol), 6-bromo-7-hydroxytetralone (24.11g, 100mmol), N,N-dimethylformamide (284mL) into the three-necked flask, stir well and add potassium carbonate ( 27.64g, 200mmol), react at room temperature for 4-6 hours, add water (284mL), ethyl acetate (284mL) after the reaction, separate the layers, extract the water layer with ethyl acetate (142mL) once more, combine the organic phases and wash with water (142mL ) twice, dried over anhydrous sodium sulfate, filtered, concentrated to remove most of the ethyl acetate, added petroleum ether (284mL) for slurry, filtered, and vacuum-dried to give Intermediate 7 (41.79g, yield 94%).
[0051] 1 HNMR (400MHz, CDCl 3 )δ7.72(d, J=2.4Hz, 1H), 7.57(s, 1H), 7.52(s, 1H), 7.49(d, J=8.4Hz, 1H), 7.17(dd, J=8.4, 2.8 Hz,1H),5.13(s,2H),2.90(t,J=6.0Hz,2H),2.67-2.61(m,2H),2.16-2.09(m,2H)
Embodiment 2
[0053]
[0054] Add compound 7 (44.45g, 100mmol) in the there-necked flask, palladium acetate (0.92g, 3mmol), XantPhos (1.68g, 3mmol), pinacol biborate (26.66g, 105mmol), potassium carbonate (41.46g, 300mmol) and N,N-dimethylacetamide (222mL), stir to dissolve, then switch to nitrogen under vacuum, heat to 60°C for 3-4 hours, add deoxygenated water (111mL) to continue the reaction at 80°C for 4-6 hours after the reaction , cooled, the mixture was added with water (111mL), then extracted 3 times with isopropyl acetate (111mL), the combined organic phases were washed 2 times with water (222mL), dried over sodium sulfate, separated by column chromatography, and concentrated to give Intermediate 1 (17.94 g, 63%). 1 HNMR (400MHz, CDCl 3 )δ7.77(d, J=8.4Hz, 1H), 7.60(s, 1H), 7.53(s, 1H), 7.35(d, J=8.4Hz, 1H), 7.17(s, 1H), 5.06( s, 2H), 3.04-2.80 (m, 2H), 2.75-2.50 (m, 2H), 2.27-2.00 (m, 2H).
Embodiment 3
[0056]
[0057] Add compound 8 (29.20g, 100mmol), 6-bromo-7-hydroxytetralone (24.11g, 100mmol) and N,N-dimethylformamide (290mL) into a three-necked flask, stir well and add potassium carbonate ( 27.64g, 200mmol), react at room temperature for 4-6 hours, add water (290mL), ethyl acetate (290mL) after the reaction, separate the layers, extract the aqueous layer with ethyl acetate (145mL) once, combine the organic phases and wash with water (145mL ) twice, dried over anhydrous sodium sulfate, filtered, concentrated to remove most of the ethyl acetate, added petroleum ether (290mL) for slurry, filtered, and vacuum-dried to obtain intermediate 9 (40.22g, yield 89%). 1 HNMR (400MHz, CDCl 3 )δ7.88(d,J=2.4Hz,1H),7.75-7.65(m,3H),7.20-7.10(m,1H),5.17(s,2H),3.05-2.75(m,2H),2.70 -2.50(m,5H),2.15-1.95(m,2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com