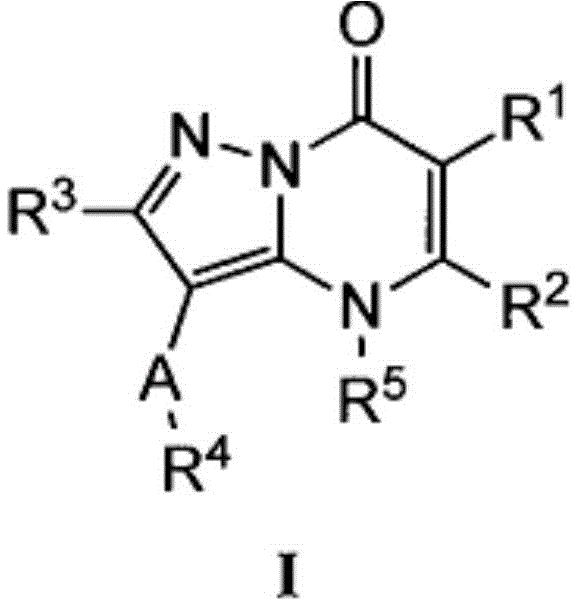
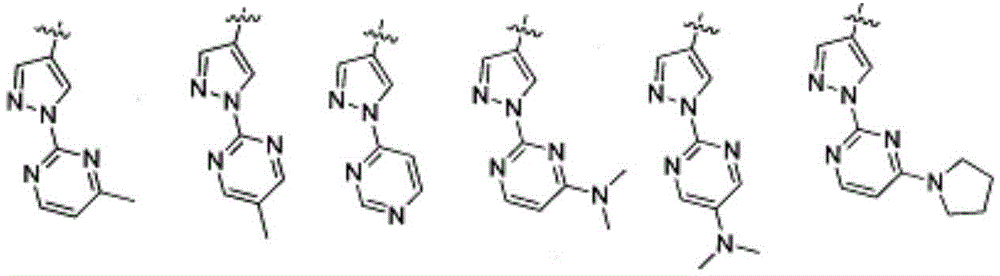
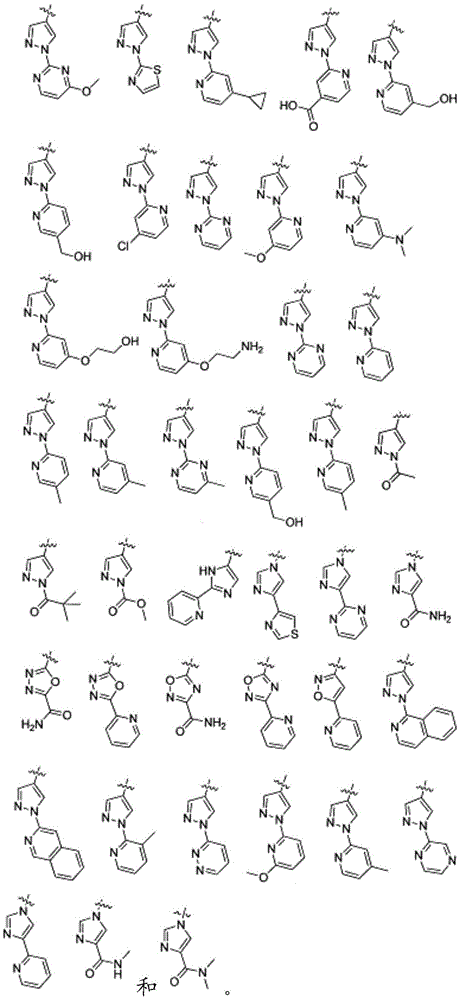
Antiproliferative compounds
一种化合物、增生性病症的技术,应用在药物组合、有机化学、抗肿瘤药等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0248] step 1
[0249]
[0250] 3-Bromo-6-ethyl-5-methylpyrazolo[1,5-a]pyrimidin-7(4H)-one
[0251] A mixture of 4-bromo-1H-pyrazol-5-amine (16.1 g, 100.0 mmol) and ethyl 2-ethyl-3-oxobutanoate (19.0 g, 120.0 mmol) in AcOH (300 mL) was heated at reflux 4h. After solvent evaporation, the residue was washed with MTBE (200 mLx3). The resulting solid was collected by filtration and dried to give the crude title compound (17.0 g, 67% yield). It was used directly in the next step without further purification. LCMS(ESI)m / z:255.9[M+H + ].
[0252] step 2
[0253]
[0254] 3-Bromo-7-chloro-6-ethyl-5-methylpyrazolo[1,5-a]pyrimidine
[0255] POCl 3 (60.8g, 400.0mmol) was added dropwise to 3-bromo-6-ethyl-5-methylpyrazolo[1,5-a]pyrimidin-7(4H)-one (25.6g, 100.0mmol) and DIPEA (51.6 g, 400.0 mmol) in toluene (500 mL) was stirred and cooled (0 °C) in the mixture. After the addition, the mixture was allowed to warm to 90 °C and stirring was continued for 4 h, at which time LC...
Embodiment 2
[0273]
[0274] 6-Ethyl-5-methyl-3-(1-(5-methylpyrimidin-2-yl)-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-7( 4H)-one
[0275] In a similar procedure to that shown in Example 1, the title compound was prepared in 15% yield from 2-chloro-5-methylpyrimidine. 1 HNMR (400MHz, DMSO-d 6 ):δ11.43(s,1H),9.06(s,1H),8.70(s,2H),8.19(s,2H),2.45-2.47(m,2H),2.40(s,3H),2.29( s,3H), 1.02-0.98(m,3H). LCMS (method C): RT = 0.721min, m / z: 335.9 [M+H + ].
Embodiment 3
[0277]
[0278] 6-Ethyl-5-methyl-3-(1-(pyrimidin-4-yl)-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidin-7(4H)-one
[0279] In a similar procedure to that shown in Example 1, the title compound was prepared in 19% yield from 4-chloropyrimidine. 1 HNMR (400MHz, DMSO-d 6 ): δ9.18(s,1H),9.11(s,1H),8.87(d,J=5.210Hz,1H),8.37(s,1H),8.22(s,1H),7.95(d,J= 5.2Hz, 1H), 2.48-2.45(m, 2H), 2.42(s, 3H), 1.03(t, J=6.8Hz, 3H). LCMS (Method C): RT = 0.713min, m / z: 321.8, 665.1 [M+H + ,2M+1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com