Polypeptide sequence capable of specifically being bound to fumonisin B1 and applications thereof
A polypeptide sequence and fumonisin technology, applied in peptides, material inspection products, biological testing, etc., can solve the problems of peptides that have not been seen yet, and achieve easy mass preparation and purification, high affinity and specificity, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
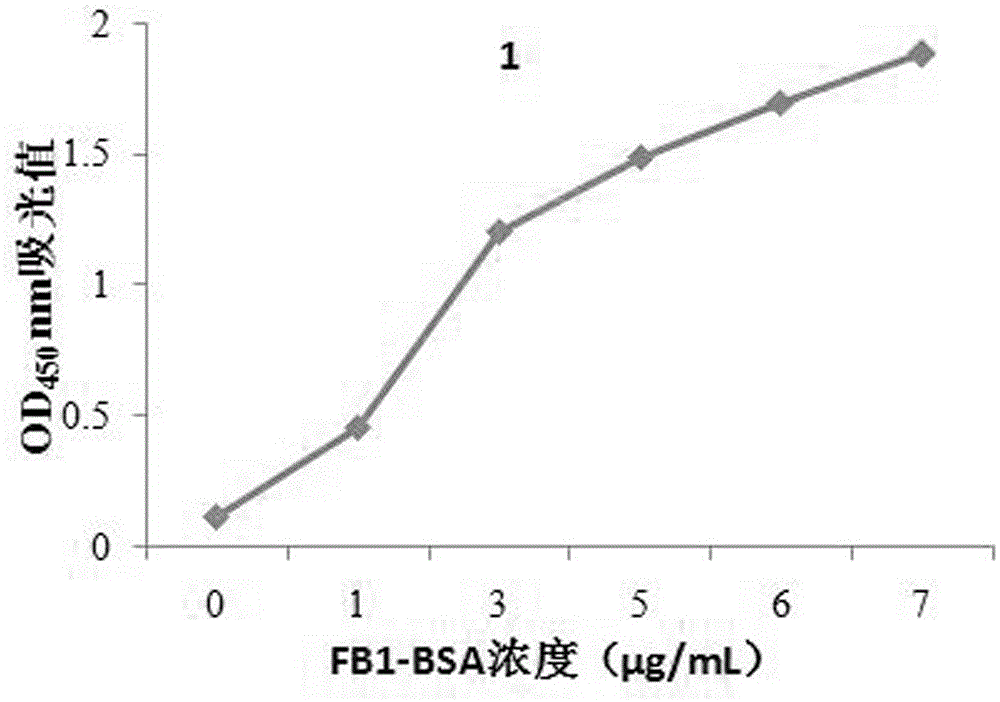
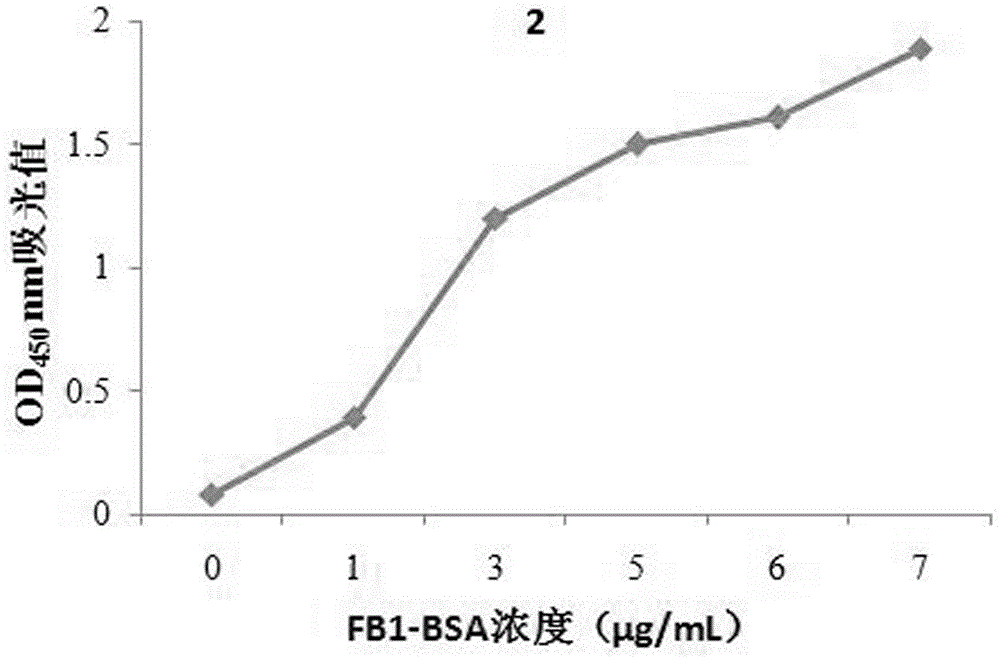
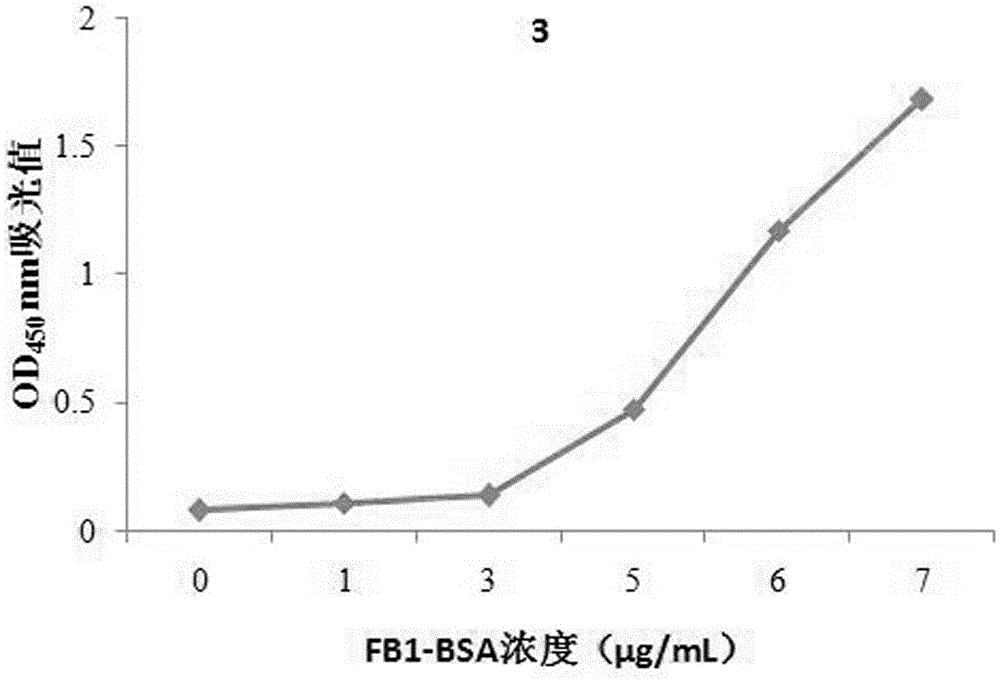
[0026] The present invention provides a combination with fumonisin B 1 A specifically binding polypeptide sequence, the polypeptide sequence is a phage display peptide, consisting of 12 amino acids, and the fumonisin B 1 There are five specific binding polypeptide sequences. Fumonisin B 1 There are five specific binding polypeptide sequences, the amino acid sequences of which are shown in SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4 and SEQ ID NO: 5.
[0027] The nucleotide sequences corresponding to the five polypeptide sequences are shown in sequence as SEQ ID NO:6, SEQ ID NO:7, SEQ ID NO:8, SEQ ID NO:9 and SEQ ID NO:10.
[0028] To achieve the above object, the present invention adopts the following technical solutions:
[0029] A and fumonisin B 1 The specific binding polypeptide sequence, the method for preparing said polypeptide is as follows:
[0030] (1) Add FB 1 -BSA is coated on the microplate, and the plate is blocked with 5% BSA, and the phage displ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com