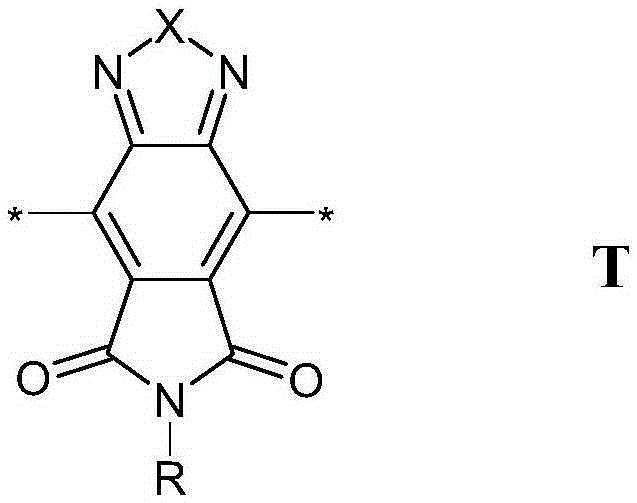
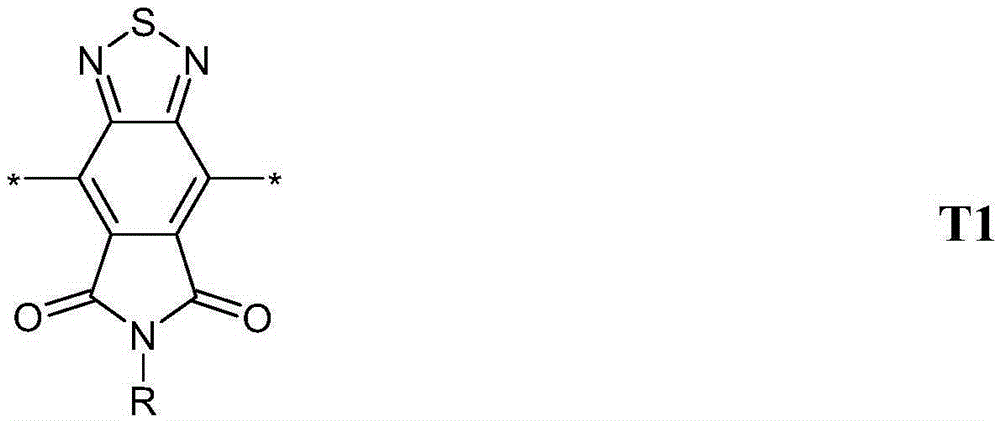Conjugated polymers
A polymer, acceptor unit technology, applied in conductive materials, conductive materials, conductors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0420] Example 1 - Polymer 1
[0421]
[0422] to 20cm 3 Add 2,6-dibromo-benzo[1,2-b; 4,5-b']dithiophene-4,8-dicarboxylate bis-dodecyl ester (309.1 mg; 0.4000 mg mol; 1.000 equiv), 2,5-bis-trimethylstannyl-thiophene (327.8 mg; 0.8000 mmol; 2.000 equiv), 4,7-dibromo-5,6-bis-octyloxy-benzene And[1,2,5]thiadiazole (165.1mg; 0.3000mmol; 0.7500eq), 4,8-dibromo-6-(2-ethyl-hexyl)-[1,2,5]thiadiazole Azolo[3,4-e]isoindole-5,7-dione (47.5mg; 0.100mmol; 0.250eq), tris(dibenzylideneacetone)dipalladium(0) (14.7mg; 0.0160mM mol; 0.0400 eq) and tri-o-tolyl-phosphine (19.5 mg; 0.0640 mmol; 0.160 eq). The vessel was emptied and purged three times with nitrogen, and degassed chlorobenzene (5.00 cm 3 ), and the reaction mixture was further degassed for 5 minutes. The reaction mixture was heated sequentially at 140°C (60 sec), 160°C (60 sec) and 170°C (1800 sec) in a microwave reactor (Initator, Biotage). Immediately after the completion of the polymerization, the reaction mixture was ...
Embodiment 2
[0423] Example 2 - Polymer 2
[0424]
[0425] to 5cm 3 Add 2,6-dibromo-benzo[1,2-b; 4,5-b']dithiophene-4,8-dicarboxylate bis-dodecyl ester (193.2 mg; 0.2500 mg mol; 1.000 equiv), 2,5-bis-trimethylstannyl-thiophene (204.9 mg; 0.5000 mmol; 2.000 equiv), 4,8-dibromo-6-(2-ethyl-hexyl)- [1,2,5]thiadiazolo[3,4-e]isoindole-5,7-dione (118.8 mg; 0.2500 mmol; 1.000 equiv), tris(dibenzylideneacetone)dipalladium (0) (9.2 mg; 0.0100 mmol; 0.040 equiv) and tri-o-tolyl-phosphine (12.2 mg; 0.0400 mmol; 0.160 equiv). The vessel was emptied and purged three times with nitrogen, and degassed chlorobenzene (2.1 cm 3 ), and the reaction mixture was further degassed for 5 minutes. The reaction mixture was heated sequentially at 140 °C (60 sec), 160 °C (60 sec) and 175 °C (1800 sec) in a microwave reactor (Initator, Biotage). Immediately after completion of the reaction, the reaction mixture was cooled to 65 °C and precipitated into stirred methanol (100 cm 3 )middle. The polymer was co...
Embodiment 3
[0426] Example 3 - Polymer 3
[0427]
[0428] to 5cm 3 Add 1,4-bis-(5-bromo-7,7-bis-(2-ethyl-hexyl)-7H-3,4-dithia-7-sila-cyclopenta[a ]cyclopentadien-2-yl)-2,3,5,6-tetrafluorobenzene (285.3 mg; 0.2500 mmol; 1.000 equivalents), 2,5-bis-trimethylstannyl-thiophene (204.9 mg; 0.5000 mmol; 2.000 equiv), 4,8-dibromo-6-(2-ethyl-hexyl)-[1,2,5]thiadiazolo[3,4-e]isoindole- 5,7-Diketone (118.8mg; 0.2500mmol; 1.000eq), tris(dibenzylideneacetone)dipalladium(0) (9.2mg; 0.0100mmol; 0.040eq) and tris-o-tolyl- Phosphine (12.2 mg; 0.0400 mmol; 0.160 equiv). The vessel was emptied and purged three times with nitrogen, and degassed chlorobenzene (2.1 cm 3), and the reaction mixture was further degassed for 5 minutes. The reaction mixture was heated sequentially at 140 °C (60 sec), 160 °C (60 sec) and 175 °C (1800 sec) in a microwave reactor (Initator, Biotage). Immediately after completion of the reaction, the reaction mixture was cooled to 65 °C and precipitated into stirred methanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com