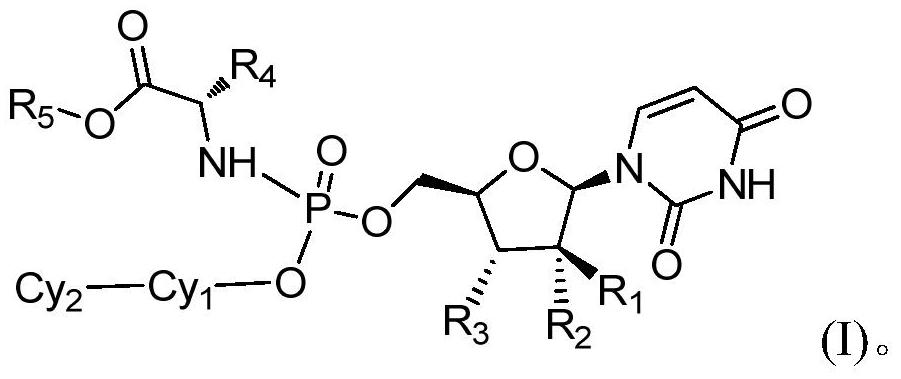
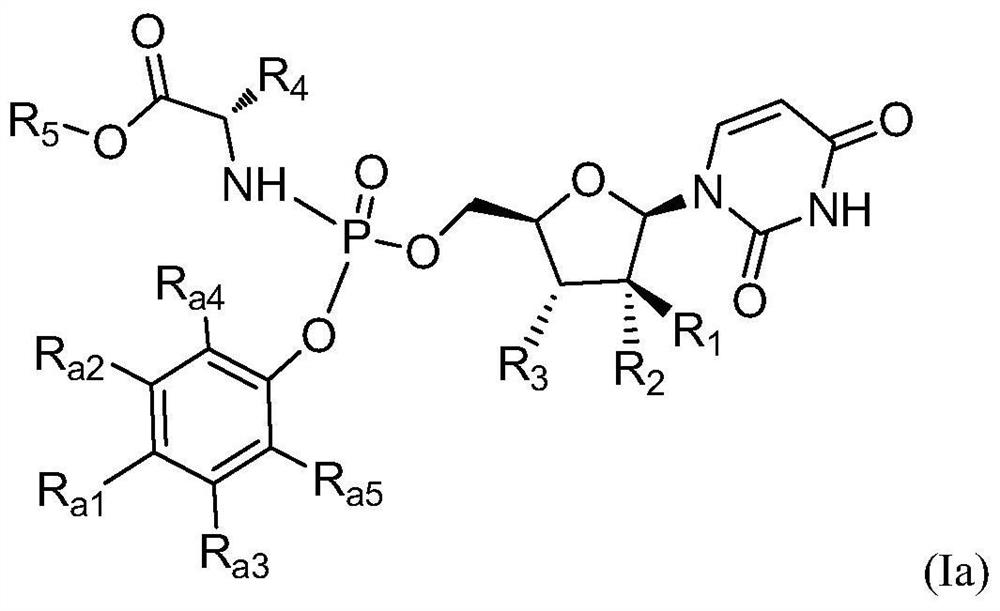
Hepatitis C virus inhibitor and its application
A compound, selected technology, applied in the field of medicinal chemistry, can solve the problems of weight loss, fatigue and weakness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Example 1 (2S)-2-(((4-(pyridin-2-yl)phenyloxy)(((2R,3R,4R,5R)-5-(2,4-dioxo-3 ,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)phosphoryl)amino)propionate isopropyl
[0169]
[0170] The preparation of step 1 4-(pyridin-2-yl)phenol
[0171] Weigh 1.38g 4-hydroxyphenylboronic acid (10mmol), 1.58g 2-bromopyridine (10mmol), 0.4g [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (Pd(dppf )Cl 2 , 0.5mmol) and 9g cesium carbonate (30mmol) in a 250mL eggplant-shaped bottle, add 50mL 1,4-dioxane and 5mL water, react at 90°C for 1.5h under the protection of argon, after the reaction, add 100mL acetic acid Extract with ethyl ester and 50 mL saturated aqueous sodium chloride solution, collect the organic phase, wash with saturated aqueous sodium chloride solution (3×50 mL), dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, and purify by column chromatography to obtain the title compound.
[0172] LC-MS...
Embodiment 2
[0179] Example 2 (2S)-2-(((4-(3-fluoropyridin-2-yl)phenyloxy)(((2R,3R,4R,5R)-5-(2,4-dioxo Isopropyl (3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)phosphoryl)amino)propionate
[0180]
[0181] Step 1 Preparation of 4-(3-fluoropyridin-2-yl)phenol
[0182] Using 4-hydroxyphenylboronic acid and 2-bromo-3-fluoropyridine as raw materials, the title compound was obtained by the same method as in Step 1 of Example 1.
[0183] LC-MS m / z:[M+H] + =190.
[0184] Step 2 (2S)-2-(((4-(3-fluoropyridin-2-yl)phenyloxy)(((2R,3R,4R,5R)-5-(2,4-dioxo -3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)phosphoryl)amino)propionic acid isopropyl preparation
[0185] 4-(3-fluoropyridin-2-yl)phenol, phosphorus oxychloride, L-alanine isopropyl hydrochloride, pentafluorophenol and (2'R)-2'-deoxy Using -2'-fluoro-2'-methyluridine as the starting material, the target compound was prepared in the same way as in Steps 2 and...
Embodiment 3
[0188] Example 3 (2S)-2-(((4-(2-fluoropyridin-4-yl)phenyloxy)(((2R,3R,4R,5R)-5-(2,4-dioxo Isopropyl (3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)phosphoryl)amino)propionate
[0189]
[0190] Preparation of step 1 4-(2-fluoropyridin-4-yl)phenol
[0191] Using 4-hydroxyphenylboronic acid and 2-fluoro-4-bromopyridine as raw materials, the title compound was obtained by the same method as in Step 1 of Example 1.
[0192] LC-MS m / z:[M+H] + =190.
[0193] Step 2 (2S)-2-(((4-(2-fluoropyridin-4-yl)phenyloxy)(((2R,3R,4R,5R)-5-(2,4-dioxo -3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)phosphoryl)amino)propionic acid isopropyl preparation
[0194] 4-(2-fluoropyridin-4-yl)phenol, phosphorus oxychloride, L-alanine isopropyl hydrochloride, pentafluorophenol and (2'R)-2'-deoxy Using -2'-fluoro-2'-methyluridine as the starting material, the target compound was prepared in the same way as in Steps 2 and...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap