Use of resveratrol-based perylene amide analogs in medicine
A technology based on peronamide and resveratrol, which is applied in the field of application of resveratrol-based peronamide analogs in the treatment of malignant tumors, and can solve the problem of unseen applications of resveratrol-based peronamide analogs And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Compound BLBB-1 preparation of
[0018] The synthetic route is as follows:
[0019]
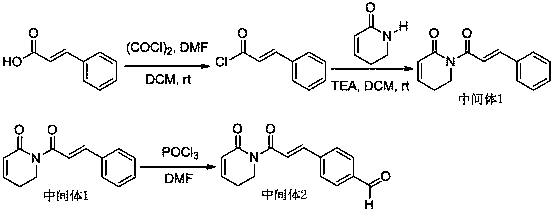
[0020] The synthetic route of intermediate 1 can be found in reference (Shoujiao Peng et al, J. Med. Chem. 2015,58, 5242−5255).
[0021] For the synthetic route of intermediate 2, please refer to the reference (Liu Wenhu et al., Acta Pharmaceutica Sinica, 2014, 49 (2): 217 −224).
[0022]
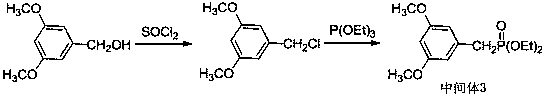
[0023] The synthetic route of intermediate 3 can be found in references (Hou Jian et al., China Journal of Pharmaceutical Industry, 2008, 39, 1, 1-8).
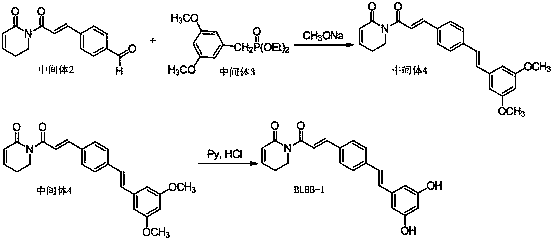
[0024] The synthetic route of compound BLBB-1 is shown in the literature (Hou Jian et al., China Journal of Pharmaceutical Industry, 2008, 39, 1, 1-8):
[0025]
[0026] Dissolve 10 mmol of intermediate 3 in 10 ml DMF under ice bath, add NaOCH 3 10 mmol and vigorously stirred, then 10 mmol of intermediate 2 was added dropwise, and reacted at room temperature for 10 h. Then it was poured into ice water, extracted with ethyl...
Embodiment 2
[0030] Example 2: Compound BLBB-2 preparation of
[0031] The synthetic route is as compound BLBB-1.
[0032] 1 H NMR (400 MHz, DMSO): δ9.50(s,1H), 9.14(s,1H), 7.92(d, J = 15.6 Hz,1H), 7.34 (d, J = 15.6 Hz,1H), 6.94 (d, J = 16.4 Hz, 1H), 6.92-6.90 (m, 3H), 6.82(d, J = 16.4 Hz, 1H), 6.52(d, J = 2.0 Hz, 2H), 6.21(t, J = 2.4 Hz, 1H), 6.01 (t, J = 9.6 Hz, 1H), 4.00 (t, J = 6.4 Hz, 2H), 3.88 (s, 6H), 2.44 (m, 2H).
[0033] MS-ESI (m / z): 421.15.
Embodiment 3
[0034] Example 3: Compound BLBB-3 preparation of
[0035] The synthetic route is as compound BLBB-1.
[0036] 1 H NMR (400 MHz, DMSO): δ9.52(s,1H), 9.12(s,1H), 7.90(d, J = 15.6 Hz,1H), 7.36 (d, J = 15.6 Hz,1H), 6.98 (d, J = 16.4 Hz, 1H), 6.94-6.92 (m, 3H), 6.80(d, J = 16.4 Hz, 1H), 6.54(d, J = 2.0 Hz, 2H), 6.22(t, J = 2.4 Hz, 1H), 6.03 (t, J = 9.6 Hz, 1H), 4.00 (t, J = 6.4 Hz, 2H), 2.42 (m, 2H).
[0037] MS-ESI (m / z): 397.11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com