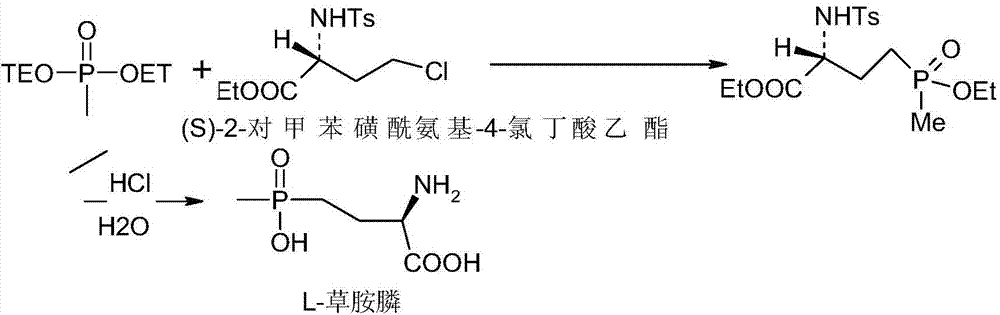
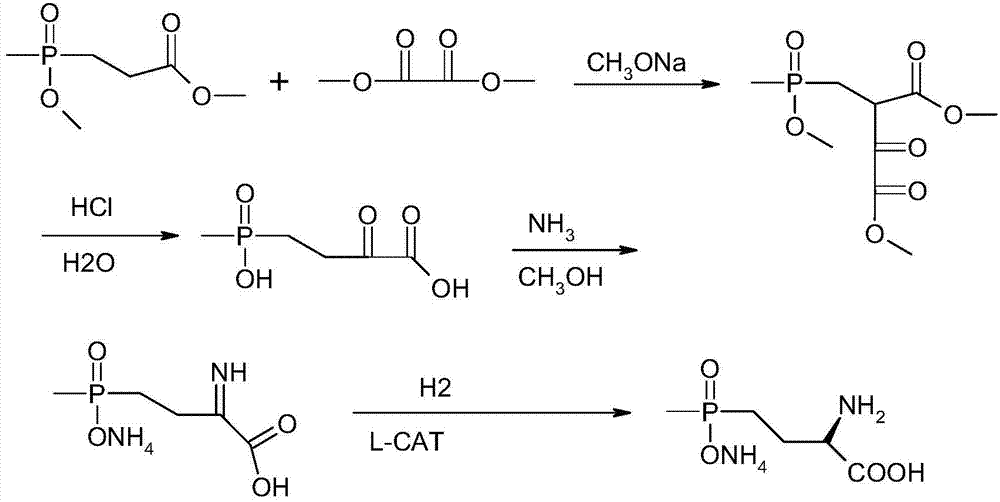
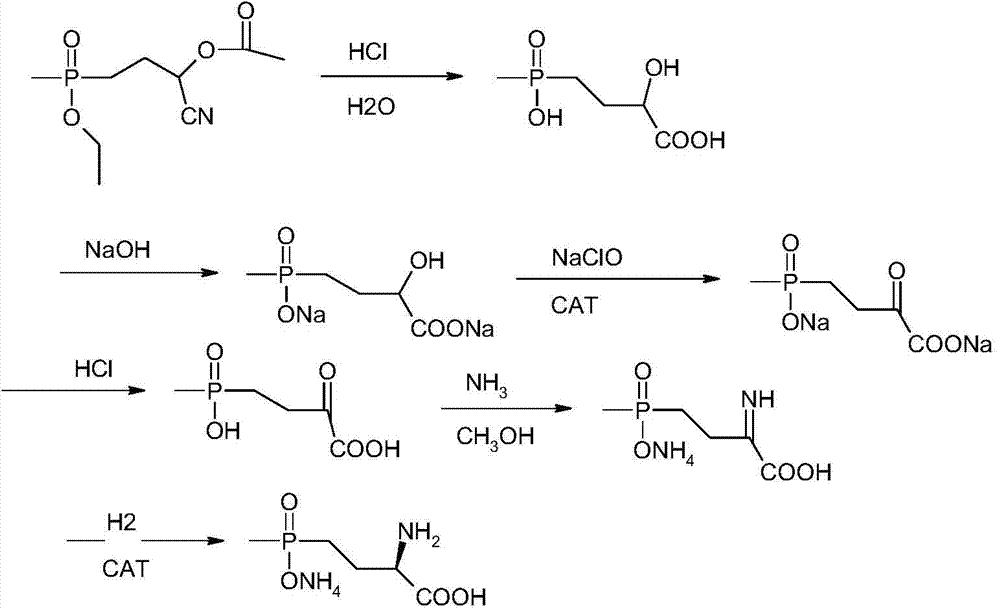
A kind of synthetic method of L-type glufosinate-ammonium ammonium salt
A synthetic method and technology of ammonium phosphonate, applied in the field of synthesis of L-type glufosinate-ammonium ammonium salt, can solve the problems of dimer formation, high price, low yield, etc., achieve simple process flow, improve weed control High efficacy and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 23.3 grams of 4-(ethoxy-(methyl)phosphinyl)-2-acetoxybutyrocyanide to 73 grams of 15% hydrochloric acid, heat and reflux at 80°C for 4 hours, and wait for the raw materials to react completely The acid water was distilled off under reduced pressure, then cooled to room temperature, methanol was added, stirred, and the ammonium chloride formed was filtered off, and the methanol filtrate was distilled off under negative pressure at 20°C. Add water and sodium hydroxide to the residue to neutralize to obtain disodium salt, then add 0.117 grams of catalyst ruthenium oxide, add dropwise 59.6 grams of 15% content of new sodium hypochlorite under stirring, control the temperature at about 30 ° C to complete the dropwise addition, and keep the temperature for 3 hours. After the oxidation reaction is basically completed, the catalyst is filtered out, most of the water is distilled off under negative pressure, then acetone is added, hydrogen chloride gas is introduced to neutra...
Embodiment 2
[0041]Add 23.3 grams of 4-(ethoxy-(methyl)phosphinyl)-2-acetoxybutyrocyanide to 73 grams of 15% hydrochloric acid, heat and reflux at 80°C for 6 hours, after the reaction of the raw materials is complete The acid water was distilled off under reduced pressure, then cooled to normal temperature, methanol was added, stirred, and the ammonium chloride formed was filtered off, and methanol was distilled off the methanol filtrate under negative pressure at 15°C. Add water and sodium hydroxide to the residue to neutralize to obtain disodium salt, then add 0.163 g of catalyst ruthenium oxide, add dropwise 74.5 g of 20% fresh sodium hypochlorite with stirring, control the temperature at about 40°C to complete the dropwise addition, and keep the temperature for 4 hours. After the oxidation reaction is basically completed, the catalyst is filtered out, most of the water is distilled off under negative pressure, then acetone is added, hydrogen chloride gas is introduced to neutralize the ...
Embodiment 3
[0043] Add 23.3 grams of 4-(ethoxy-(methyl)phosphinyl)-2-acetoxybutyrocyanide to 146 grams of 10% hydrochloric acid, heat and reflux at 100°C for 8 hours, and wait for the raw materials to react completely The acid water was distilled off under reduced pressure, then cooled to normal temperature and methanol was added, stirred, and the ammonium chloride formed was filtered off, and the methanol filtrate was distilled off methanol under negative pressure at 20°C. Add water and sodium hydroxide to the residue to neutralize to obtain disodium salt, then add 0.186 grams of catalyst ruthenium oxide, add 149.2 grams of 15% fresh sodium hypochlorite dropwise under stirring, control the temperature at about 50°C to complete the dropwise addition, and keep the temperature for 5 hours. After the oxidation reaction is basically completed, the catalyst is filtered out, most of the water is distilled off under negative pressure, then acetone is added, hydrogen chloride gas is introduced to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com