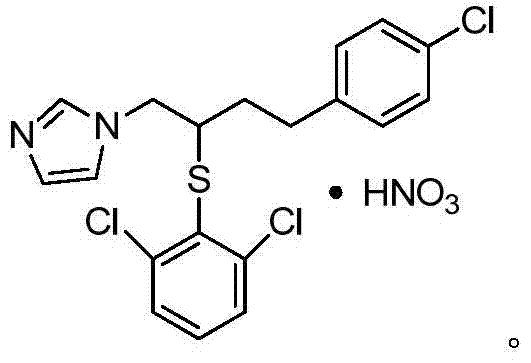
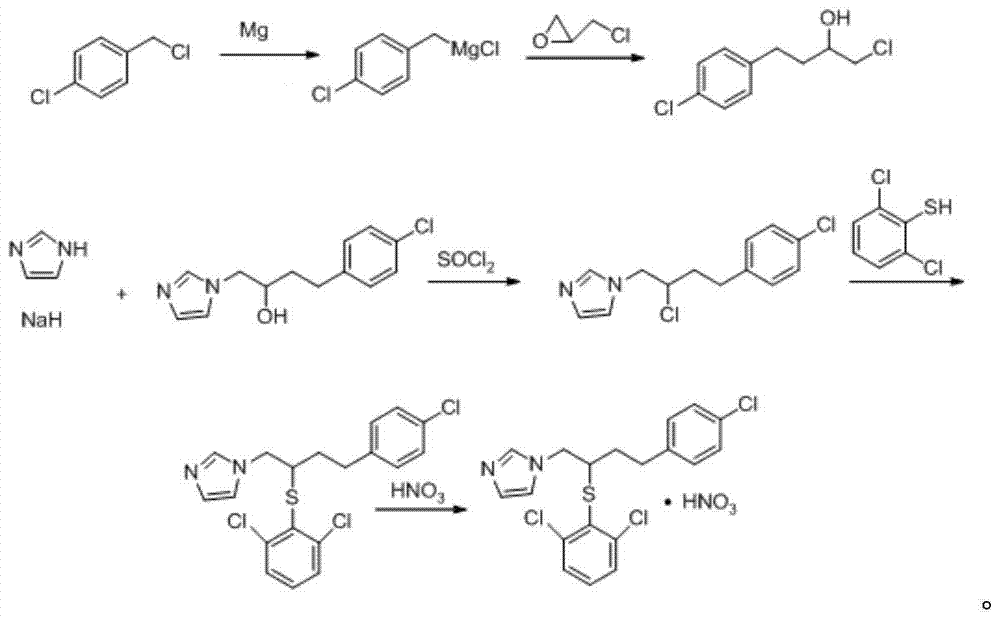
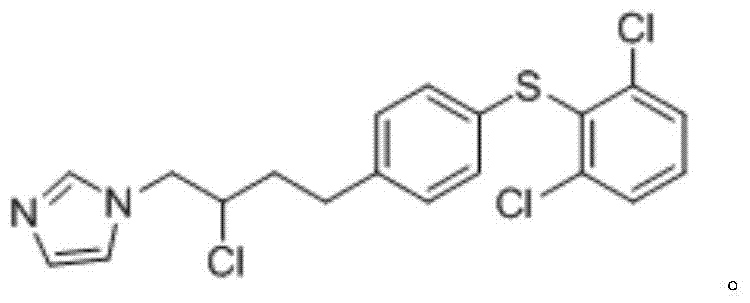
A kind of method of industrialized production butoconazole nitrate
A technology for butconazole nitrate and imidazole, applied in the field of compound synthesis technology, can solve problems such as product yield and purity that cannot meet industrialized production, complicated post-processing steps, effective control and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Follow the steps below to produce butoconazole nitrate:
[0067] (1) Take 5kg 1-(2-chloro-4-(4-chlorophenyl)butyl)-1hydro-imidazole, 5kg 2,6-dichlorothiophenol, 2.5kg anhydrous potassium carbonate and 40kg acetone , reflux at 60°C for 5 hours, add 0.8kg of anhydrous potassium carbonate, reflux at 60°C for 7 hours, cool slowly with circulating water at 0-4°C, centrifuge at 2825r / min for 20min, discard the solid , retain the liquid, which is the filtrate; concentrate the filtrate at 60°C until no acetone is evaporated to obtain a concentrate;
[0068] (2) Take the concentrate obtained in step (1), add an extract composed of diethyl ether and water with a weight ratio of 2.5:1.5, after sufficient extraction, discard the aqueous phase, and leave the organic phase for subsequent use;
[0069] (3) Under ice-bath and stirring condition, in step (2) gained organic phase, dropwise add concentrated nitric acid, filter after stopping to generate precipitation; Discard filtrate, u...
Embodiment 2
[0072] Follow the steps below to produce butoconazole nitrate:
[0073] (1) Take 4kg 1-(2-chloro-4-(4-chlorophenyl) butyl)-1 hydrogen-imidazole, 4kg 2,6-dichlorothiophenol, 1kg anhydrous potassium carbonate and 30kg acetone, Reflux reaction at 55°C for 4.5h, add 0.5kg of anhydrous potassium carbonate, reflux reaction at 55°C for 6.5 hours, slowly cool with circulating water at 0-4°C, centrifuge at 2825r / min for 10min, discard the solid, The retained liquid is the filtrate; the filtrate is concentrated at 55°C until no acetone is evaporated to obtain the concentrate;
[0074] Steps (2), (3) are the same as in Example 1.
[0075] After testing, the yield of the product in this example was 89.58%. The target product and impurity content were detected by HPLC and standard substances. After testing, the content of the target product 1-(4-(4-chlorophenyl)-2-((2,6-dichlorophenyl)thio)butyl)-1 hydrogen-imidazole mononitrate was 91.44% , the content of the impurity 1-(2-chloro-4-(4...
Embodiment 3
[0077] Follow the steps below to produce butoconazole nitrate:
[0078] (1) Get 6kg 1-(2-chloro-4-(4-chlorophenyl) butyl)-1 hydrogen-imidazole, 6kg 2,6-dichlorothiophenol, 4kg anhydrous potassium carbonate and 50kg acetone, Reflux reaction at 65°C for 5.5 hours, add 1 kg of anhydrous potassium carbonate, reflux reaction at 65°C for 7.5 hours, slowly cool with circulating water at 0-4°C, centrifuge at 2825r / min for 30min, discard the solid, keep The liquid is the filtrate; the filtrate is concentrated at 65°C until no acetone is evaporated to obtain a concentrate;
[0079] Steps (2), (3) are the same as in Example 1.
[0080] After testing, the yield of the product in this example was 95.3%. The target product and impurity content were detected by HPLC and standard substances. After testing, the content of the target product 1-(4-(4-chlorophenyl)-2-((2,6-dichlorophenyl)thio)butyl)-1 hydrogen-imidazole mononitrate is 92.25% , the content of the impurity 1-(2-chloro-4-(4-((2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com