C3, C6, C17-trisubstituted dammarane type triterpenoid saponin derivative, pharmaceutical composition thereof and applications of derivative in pharmacy
A technology of dammarane type and triterpenoid saponins, applied in the field of medicine, can solve the problems of no neurodegenerative diseases, no pharmacological activity of dammarane type triterpenoid saponins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
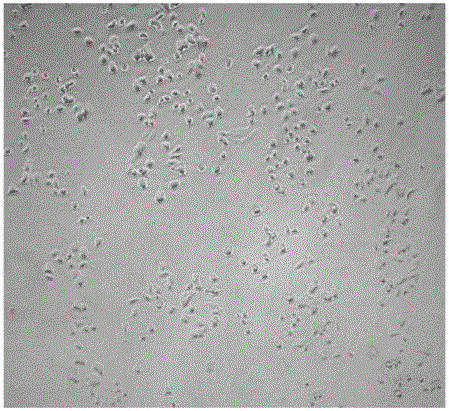
[0034] (3β,12β,20S,22E,24S)-3,12,20,24,25-pentahydroxydammar-22-ene-3-O-β-D-glucopyranosyl-(1→2)- Evaluation of the activity of β-D-glucopyranoside in promoting the differentiation of PC12 cells:
[0035]
[0036] (3β,12β,20S,22E,24S)-3,12,20,24,25-pentahydroxydammar-22-ene-3-O-β-D-glucopyranosyl-(1→2)- β-D-glucopyranoside
[0037] [(3β,12β,20S,22E,24S)-3,12,20,24,25-pentahydroxydammar-22-ene-3-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside ]
[0038] A new compound [(3β,12β,20S,22E,24S)-3,12,20,24,25-pentahydroxydammar-22-ene-3-O-β-D-glucopyranosyl-(1→ 2) Preparation of -β-D-glucopyranoside]: After the steamed Panax notoginseng (15Kg) was pulverized, it was extracted three times with 80% methanol water under reflux, each time for 3 hours. The organic solvent was evaporated to obtain about 3 kg of extract, which was dissolved in water and then subjected to column chromatography on a Diaion 101 column (250 × 30 cm). The polysaccharides were removed by washing with water,...
Embodiment 2
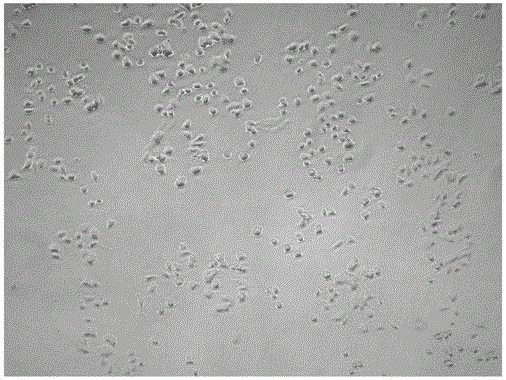
[0043] (3β,12β,20S,22E,24R)-3,12,20,24,25-pentahydroxydammar-22-ene-3-O-β-D-glucopyranosyl-(1→2)- Evaluation of β-D-glucopyranoside's ability to promote differentiation of PC12 cells
[0044]
[0045] (3β,12β,20S,22E,24R)-3,12,20,24,25-pentahydroxydammar-22-ene-3-O-β-D-glucopyranosyl-(1→2)- β-D-glucopyranoside
[0046] [(3β,12β,20S,22E,24R)-3,12,20,24,25-pentahydroxydammar-22-ene-3-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside ]
[0047] New compound (3β,12β,20S,22E,24R)-3,12,20,24,25-pentahydroxydammar-22-ene-3-O-β-D-glucopyranosyl-(1→2 Preparation of )-β-D-glucopyranoside: After the boiled Panax notoginseng (15Kg) was pulverized, it was extracted three times with 80% methanol water under reflux, each time for 3 hours. The organic solvent was evaporated to obtain about 3 kg of extract, which was dissolved in water and then subjected to column chromatography on a Diaion 101 column (250 × 30 cm). The polysaccharides were removed by washing with water, and then rinsed wi...
Embodiment 3
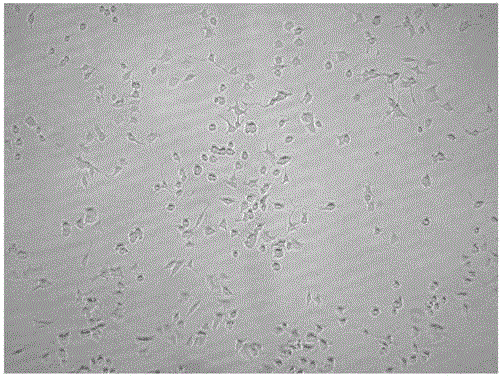
[0051] [(3β,6α,12β,20E,23R,24S)-3,6,12,23,24,25-hexahydroxydammar-20(22)-ene-6-O-β-D-glucopyranose Evaluation of Glycosides Promoting Differentiation Activity of PC12 Cells:
[0052]
[0053] (3β,6α,12β,20E,23R,24S)-3,6,12,23,24,25-hexahydroxydammar-20(22)-ene-6-O-β-D-glucopyranoside [(3β,6α,12β,20E,23R,24S)-
[0054] 3,6,12,23,24,25-hexahydroxydammar-20(22)-ene-6-O-β-D-glucopyranoside]
[0055] (3β,6α,12β,20E,23R,24S)-3,6,12,23,24,25-hexahydroxydammar-20(22)-ene-6-O-β-D-glucopyranoside Preparation: After the steamed notoginseng (15Kg) was pulverized, it was extracted three times with 80% methanol water under reflux, each time for 3 hours. The organic solvent was evaporated to obtain about 3 kg of extract, which was dissolved in water and then subjected to column chromatography on a Diaion 101 column (250 × 30 cm). The polysaccharides were removed by washing with water, and then rinsed with methanol to obtain the total saponins of Panax notoginseng (1.68 kg). The total ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com