Medicine composition for treating macular oedema
A technology of macular edema and composition, which is applied in the direction of drug combination, pharmaceutical formula, plant raw materials, etc., can solve the problems of retinal thickness increase, macular edema, affecting vision recovery, etc., and achieve the effect of delaying and preventing the formation and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: The pharmaceutical composition and preparation method thereof for the treatment of macular edema
[0034] The composition and parts by weight of the crude drug of the pharmaceutical composition for the treatment of macular edema are:
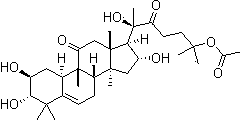
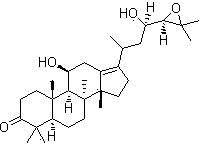
[0035] 95 parts by weight of anarch root, 75 parts by weight, 75 parts by weight, 75 parts by weight, 55 parts by weight, of rosette thistle, 35 parts by weight, 4.5 parts by weight, 4.5 parts by weight, of alisatiol B;
[0036] Preparation:
[0037] (1) According to the ratio of the raw materials, take the glycosides, styloides, anarchigen, and rosettes, mix them evenly, use ethanol with a concentration of 50% by weight as a solvent, and extract them by warm soaking at 60°C, and the number of extractions is 5 times , each extraction time is 1 hour, each time the amount of solvent is 15 times of the total weight of the above-mentioned medicinal materials, filtered, the extract is recovered from ethanol, concentrated to a re...
Embodiment 2
[0039] Embodiment 2: the pharmaceutical composition for the treatment of macular edema and its preparation method
[0040] The composition and parts by weight of the crude drug of the pharmaceutical composition for the treatment of macular edema are:
[0041] 90 parts by weight of anarch root, 80 parts by weight of glycopyrrolate, 50 parts by weight of rosette flower 50 parts by weight, 40 parts by weight of podocarpus resinol, 3 parts by weight of alisatiol B2;
[0042] Preparation:
[0043] (1) According to the ratio of the raw materials, take the glycosides, styloides, anarchigen, and rosettes, mix them evenly, use ethanol with a concentration of 50% by weight as a solvent, and extract them by warm soaking at 60°C, and the number of extractions is 5 times , each extraction time is 1 hour, each time the amount of solvent is 15 times of the total weight of the above-mentioned medicinal materials, filtered, the extract is recovered from ethanol, concentrated to a relative den...
Embodiment 3
[0045] Embodiment 3: the pharmaceutical composition and preparation method thereof for the treatment of macular edema
[0046] The composition and parts by weight of the crude drug of the pharmaceutical composition for the treatment of macular edema are:
[0047] 100 parts by weight of anarch root, 70 parts by weight of glycopyrrolate, 60 parts by weight of rosette flower, 30 parts by weight of podocarpus resinol, 6 parts by weight of alisatiol B1 by weight;
[0048] Preparation:
[0049] (1) According to the ratio of the raw materials, take the glycosides, styloides, anarchigen, and rosettes, mix them evenly, use ethanol with a concentration of 50% by weight as a solvent, and extract them by warm soaking at 60°C, and the number of extractions is 5 times , each extraction time is 1 hour, each time the amount of solvent is 15 times of the total weight of the above-mentioned medicinal materials, filtered, the extract is recovered from ethanol, concentrated to a relative density...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap