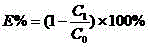Preparation method of chitosan direct quaternary ammonium salt dyeing and finishing low-salt assistant
A chitosan, quaternary ammonium salt technology, applied in dyeing, textiles and papermaking, etc., can solve the problems of hydrophilic performance, toxic and side effects, and limited increase in reactive dyes dyeing rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Put 1.0g of chitosan (molecular weight: 50,000) in a three-necked flask, and add 40mL of N-methylpyrrolidone, 2.65g of potassium iodide, 5.5mL of 15% sodium hydroxide solution, and 5.75mL of methyl iodide into the flask. The flask was placed in a stirrer and stirred rapidly, the mixture turned pale yellow. Stir and reflux in a water bath at 60° C. for 75 min. After the reaction is complete, a golden yellow solution is obtained. Pour the resulting solution into 40 mL of 5% sodium chloride solution to exchange iodide ions, and stir for 120 min. It was then poured into a large amount of absolute ethanol and stirred. The mixture was placed in a refrigerator at 4°C for precipitation overnight, and the supernatant was discarded to obtain a light yellow precipitate. The precipitate was washed three times with a large amount of absolute ethanol and petroleum ether, and centrifuged for 10 minutes at a speed of 3000 rpm. The product was vacuum-dried in a vacuum drying oven to ...
Embodiment 2
[0018] The first step of synthesis: Weigh 1.0g of chitosan (molecular weight: 50,000) and place it in a three-necked flask, add N-methylpyrrolidone 40mL, potassium iodide 2.65g, 15% sodium hydroxide solution 5.5mL, methyl iodide 5.75mL. The flask was placed in a stirrer and stirred rapidly, the mixture turned pale yellow. Stir and reflux in a water bath at 60° C. for 75 min. After the reaction is complete, a golden yellow solution is obtained. Pour the resulting solution into a large amount of absolute ethanol and stir, put it in a refrigerator at 4°C to precipitate overnight, pour off the supernatant to obtain a light yellow precipitate, wash the precipitate with a large amount of absolute ethanol 3 times, and centrifuge for 10 minutes , the speed was 3000 rpm, and the product was dried in a vacuum oven to obtain a pale yellow solid precipitate. The second step of synthesis: the product obtained in the first step of synthesis was dissolved in 40 mL of N-methylpyrrolidone an...
Embodiment 3
[0020] Repeat the first step of the experiment in group A2 to synthesize. The second step of synthesis: Add 40mL of N-methylpyrrolidone, heat to 60°C, then add 2.65g of potassium iodide, 3.5mL of iodomethane, and 5.5mL of 15% sodium hydroxide solution, stir rapidly, and heat, stir and reflux in a water bath at 60°C for 240min After the reaction, the product was poured into 40 mL of 5% sodium chloride solution to exchange iodide ions, and the resulting solution was added dropwise to a large amount of acetone, stirred rapidly, precipitated overnight, and the supernatant was discarded. The precipitate was washed three times with a large amount of absolute ethanol and petroleum ether respectively, and centrifuged for 10 min at a speed of 3000 rpm. The obtained precipitate was vacuum-dried in a vacuum drying oven to obtain chitosan quaternary ammonium salt group A3.
[0021] The above-mentioned sample adopts the following method to test its low-salt dyeing effect for cotton fabric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com