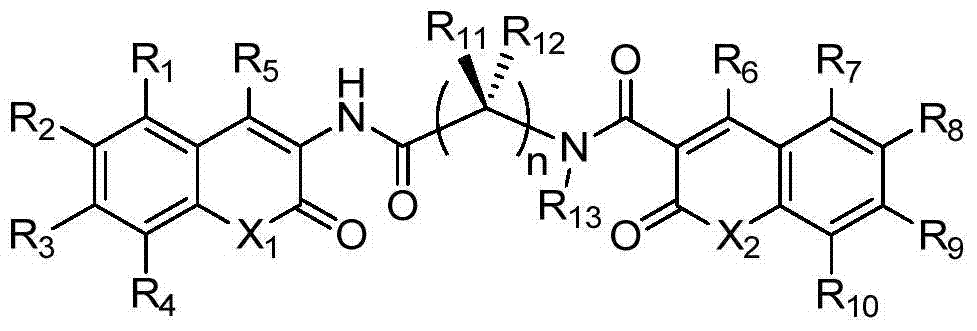
A class of dicoumarins and preparation method and use thereof
A dicoumarin and compound technology, which is applied in the direction of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., can solve the problems of inability to comprehensively examine the influence of linking partial kinase inhibitory activity, and the difficulty of compound synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
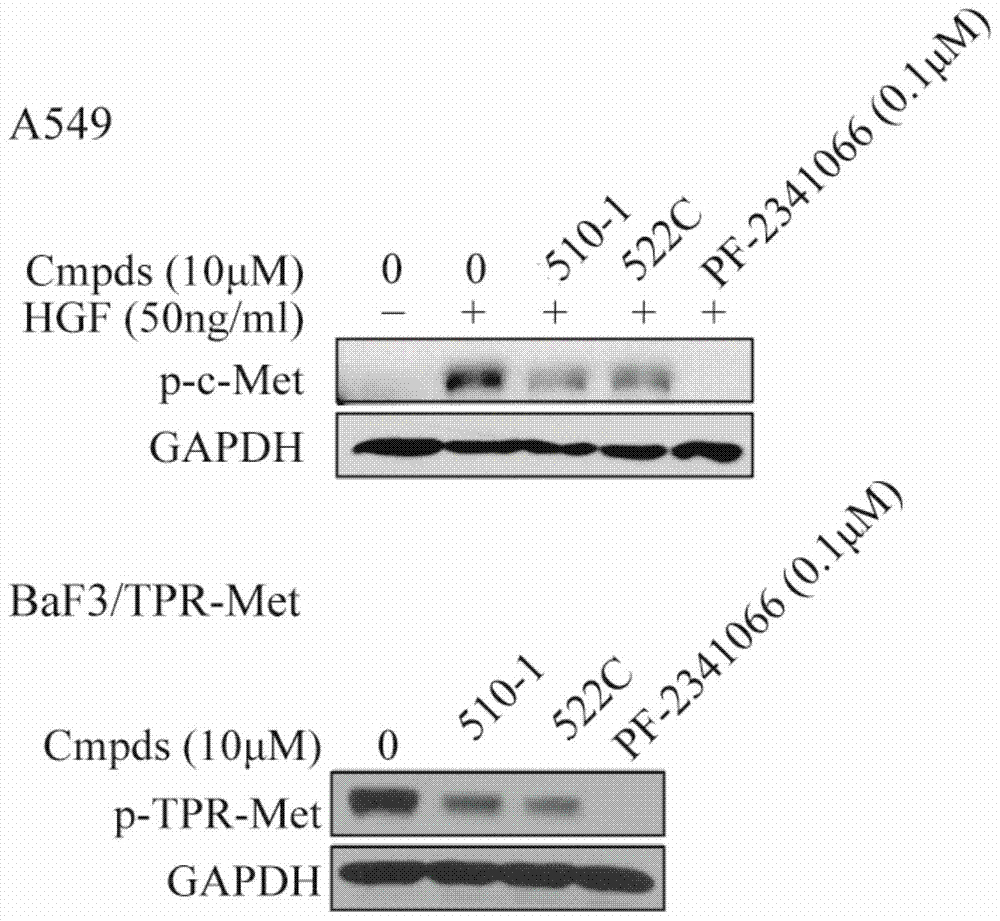
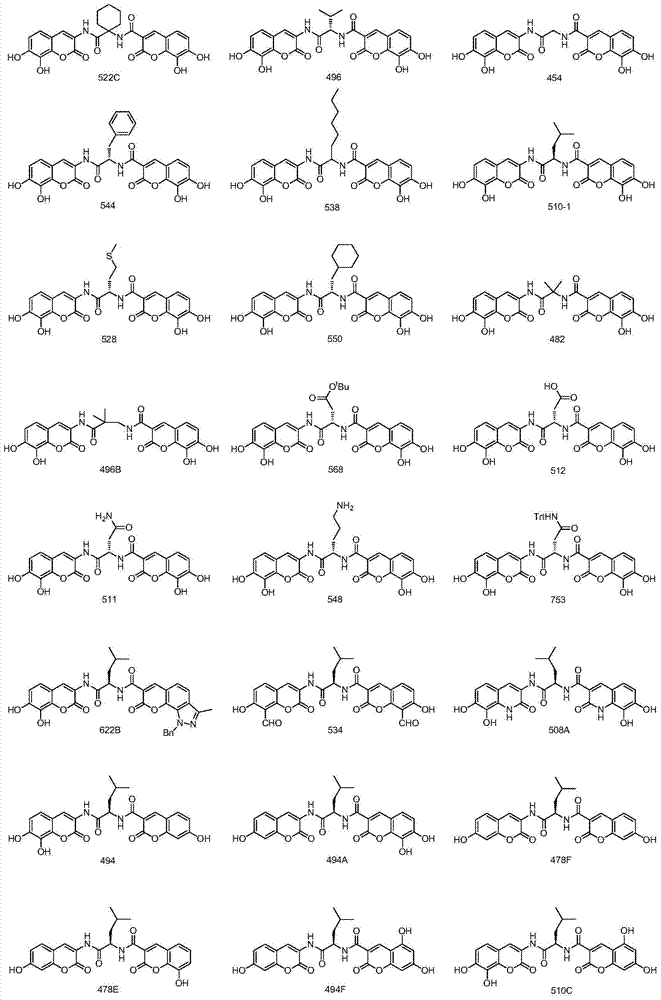
[0039] (R)-N-(N-(7,8-dihydroxycoumarin-3-)-4-methyl-pentanamide-2-)-7,8-dihydroxycoumarin-3-amide ( Compound No.: 510-1)
[0040]
[0041] 7,8-Bis(methoxymethoxy)-coumarin-3-amine (120 mg, 0.427 mmol) (prepared by reference method: Sivakumar, Krishnamoorthy; Xie, Fang; Cash, Brandon M.; Long, Su ;Barnhill,Hannah N.;Wang,Qian.Organic Letters,2004,vol.6,#24,p.4603-4606) and N-(9-fluorenylmethoxycarbonyl)-D-leucine (Fmoc-D -Leu-OH) (227 mg, 0.641) was dissolved in 5 mL of 30% pyridine / dichloromethane, added 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI), stirred at room temperature for 24 After 1 hour, the solvent was spun off, and 190 mg of intermediate 1 was obtained as a white solid by column chromatography, with a molar yield of 72%. 1 H NMR (300MHz, CDCl 3 )δ8.60(s,1H),8.57(br,1H),7.75(d,J=7.5Hz,2H),7.62-7.56(m2H),7.38(t,J=7.5Hz,2H),7.30( (t,J=7.2Hz,2H),7.18(d,J=8.7Hz,1H),7.13(d,J=8.7Hz,1H),5.32-5.18(m,5H),4.48(d,J= 6.0Hz,2H),4.38(s,1H),4.23(t,J=6....
preparation Embodiment 2
[0046] N-(1-((7,8-dihydroxycoumarin-3-)carbamoyl)cyclohexyl)-7,8-dihydroxycoumarin-3-amide (compound number: 522C)
[0047]
[0048] Referring to the method of Preparation Example 1, with 7,8-bis(methoxymethoxy)-coumarin-3-amine, 1-(9-fluorenylmethoxycarbonyl-amino)cyclohexylcarboxylic acid and 7, The target compound was prepared from 8-bis(methoxymethoxy)-coumarin-3-carboxylic acid as the starting material. 1 H NMR (300MHz, DMSO-d 6)δ10.73(br s,1H),9.94(br s,1H),9.61(br s,1H),9.31(br s,1H),9.09(s,1H),9.03(s,1H),8.72 (s,1H),8.38(s,1H),7.32(d,J=8.4Hz,1H),7.01(d,J=9.0Hz,1H),6.93(d,J=8.4Hz,1H),6.82 (d,J=8.1Hz,1H),2.28-2.18(m,2H),1.88-1.74(m,2H),1.72-1.56(m,3H),1.54-1.36(m,2H),1.35-1.20 (m,1H). 13 C NMR (75MHz, DMSO-d 6 )δ173.0, 161.5, 161.4, 158.0, 152.4, 148.9, 148.0, 144.2, 140.0, 132.1, 131.9, 126.4, 121.6, 120.3, 118.0, 113.8, 113.2 (double carbon), 112.1, 111.8), 59.2.8 (3 double carbon), ,24.71,20.92 (double carbon).
preparation Embodiment 3
[0050] (S)-N-(N-(7,8-dihydroxycoumarin-3-)-3-methyl-butyramide-2-)-7,8-dihydroxycoumarin-3-amide ( Compound No.: 496)
[0051]
[0052] Referring to the method of Preparation Example 1, with 7,8-bis(methoxymethoxy)-coumarin-3-amine, N-(9-fluorenylmethoxycarbonyl)-L-valine and 7, The target compound was prepared from 8-bis(methoxymethoxy)-coumarin-3-carboxylic acid as the starting material. 1 H NMR (300MHz, DMSO-d 6 )δ10.65(s,1H),9.94(s,1H),9.90(s,1H),9.60(s,1H),9.31(s,1H),9.13(d,J=8.7Hz,1H), 8.78(s,1H),8.46(s,1H),7.34(d,J=8.7Hz,1H),7.00(d,J=8.7Hz,1H),6.91(d,J=8.7Hz,1H), 6.81(d,J=8.4Hz,1H),4.97-4.90(m,1H),2.27-2.08(m,1H),1.05-0.85(m,6H). 13 C NMR (75MHz, DMSO-d 6 )δ170.89,161.5,161.2,157.7,152.3,149.1,148.1,144.2,140.3,132.0,131.9,127.9,121.5,120.1,118.1,113.6,113.0,112.8,112.1,117,111.94.3,57
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com