Preparation method of high-purity apremilast B crystal form
A high-purity, crystal-form technology, applied in the field of medicine, can solve problems such as low purity, and achieve the effects of safety, simplicity, strong operability, and easy industrial implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
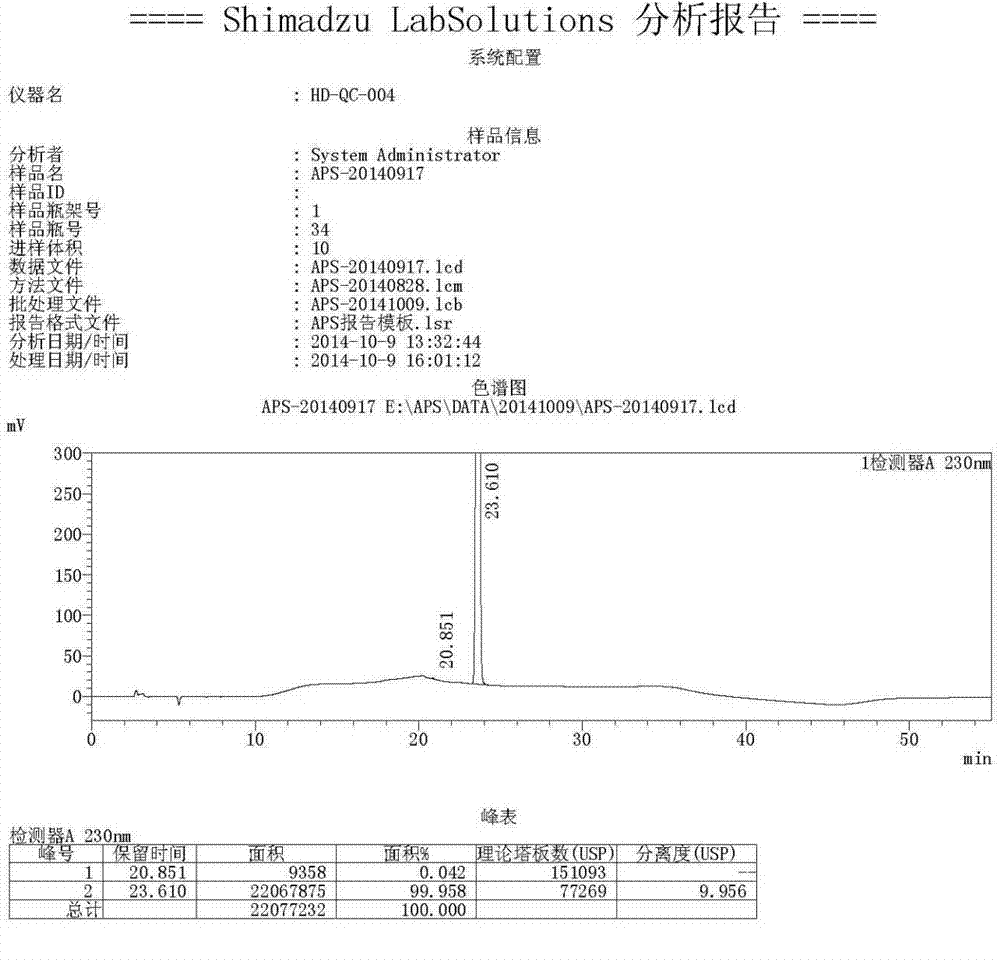
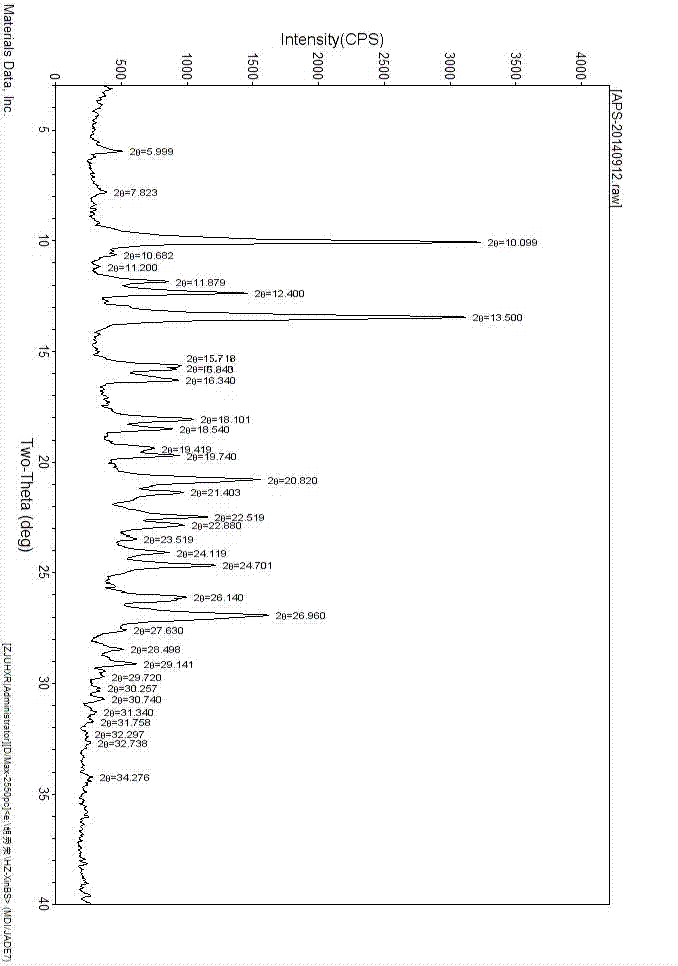
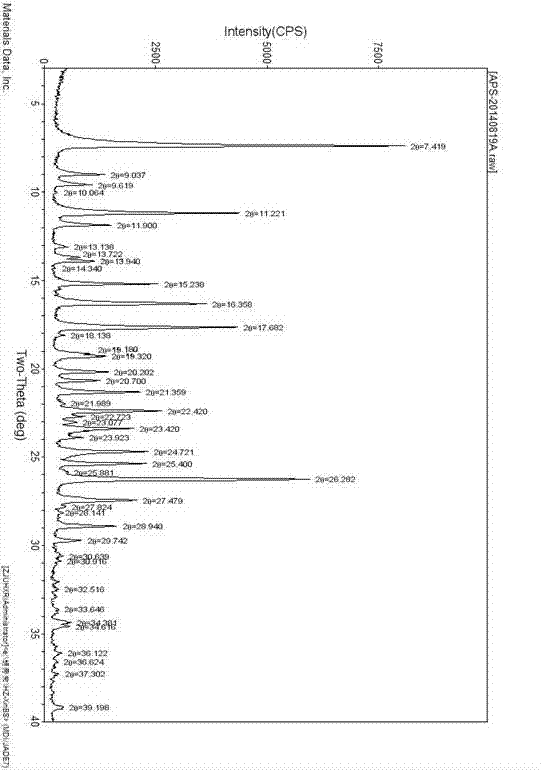
[0025] Add 2000 mL of acetic acid and 400.0 g of (S)-2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulfonyl)-ethan-2-ylamine to a 10 L three-necked flask in sequence - N-acetyl-L-leucine salt and 220.6 g of 3-acetylaminophthalic anhydride, heated to reflux for 4 hours. Cool in a water bath and filter. After distilling off the solvent, add 4000mL of ethyl acetate, followed by 4000mL of water and 4000mL of saturated NaHCO 3 Solution wash. The organic phase was transferred to a 10 L three-necked flask, and 3500 mL of toluene was added dropwise. After the dropwise addition was completed, stir for 30 min and filter. The filter cake was air-dried in a drying oven at 80°C to obtain 391g of a light yellow solid, i.e. Apremilast C crystal form (the reflection angle 2θ of the X-ray powder diffraction pattern of the obtained crystal is at 7.5°, 11.3°, 16.4°, 17.8° , there is a characteristic peak at 26.4°, which is confirmed to be the crystal form of Apremilast C, see the attached figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com