Synthesis method of morpholine compounds
A synthesis method and compound technology, which is applied in the field of synthesis of morpholine compounds, can solve problems such as poor environmental protection, and achieve the effects of low cost, mild reaction conditions, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
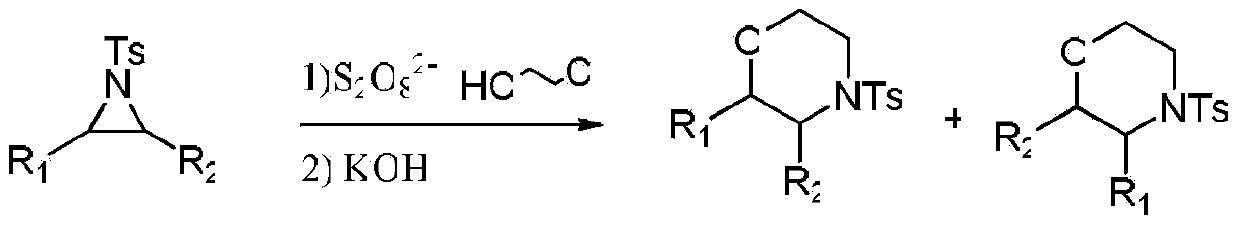
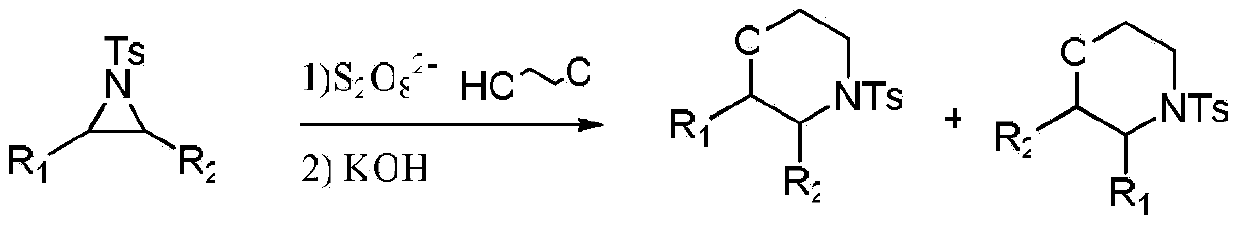
[0015] Specific embodiment one: the synthetic method of a kind of morpholine compounds of the present embodiment is carried out through the following steps:
[0016] 1. Add ring nitrogen compound, persulfate and 2-chloroethanol into the reaction vessel, react at room temperature and stirring speed of 400r / min~600r / min for 0.5h~24h to obtain the reaction liquid; The molar ratio of the ring nitrogen compound to the persulfate is 1: (1-2); the molar ratio of the ring nitrogen compound to 2-chloroethanol is 1: (5-10);
[0017] 2. Add a diluent to the reaction solution obtained in step 1 to dilute, then add KOH for cyclization reaction at room temperature for 1h to 2h, then filter, and then concentrate to dryness to obtain a solid; the ring nitrogen described in step 1 The ratio of the molar amount of the compound to the volume of the diluent described in step 2 is 1 mmol: (10-20) mL; the molar ratio of the ring nitrogen compound described in step 1 to the KOH described in step 2 i...
specific Embodiment approach 2
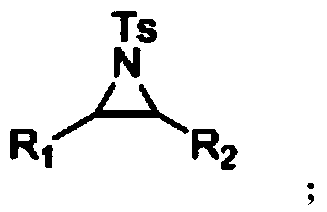
[0024] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that the general structural formula of the ring nitrogen compound described in step 1 is where R 1 is aryl, chain or cyclic alkyl, R 2 Aryl, chain or cyclic alkyl. Other steps and parameters are the same as those in the first embodiment.
specific Embodiment approach 3
[0025] Specific embodiment three: this embodiment is different from specific embodiment one or two in that: the persulfate described in step one is sodium persulfate, potassium persulfate or ammonium persulfate. Other steps and parameters are the same as those in Embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com