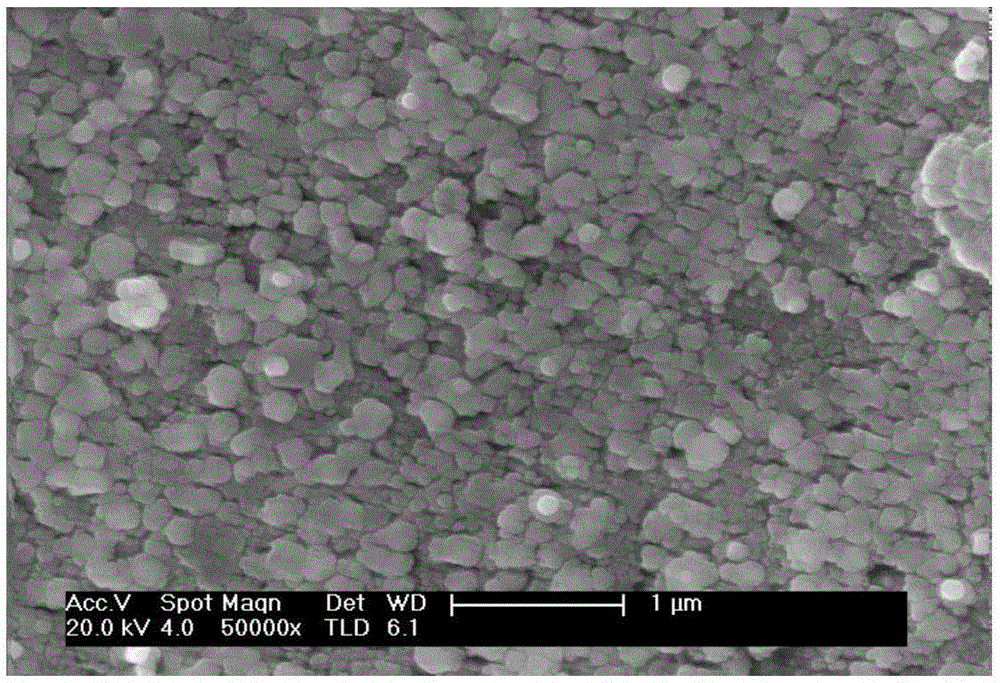
Monolithic catalyst for catalytic combustion of methane and preparation method thereof
A monolithic catalyst, methane catalytic combustion technology, used in fuel additives, fuel, petroleum industry and other directions, can solve the problems of difficult recovery of solution, difficulty in accurately controlling precious metal loading, waste of resources, etc. Smooth appearance and process controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of palladium sol takes palladium nitrate and citric acid as raw materials, and dilute nitric acid and distilled water as solvents.
[0042] The preparation steps are as follows:
[0043] (1) Weigh 100mg of Pd(NO 3 ) 2 ﹒ 2H 2 O was dissolved in 15ml of 10% dilute nitric acid by volume fraction to obtain solution A.
[0044] (2) Weigh 200 mg of citric acid and dissolve it in 10 ml of water to obtain solution B.
[0045] (3) Mix solution A prepared in step (1) with solution B prepared in step (2), add concentrated NH 3 ﹒ 2H 2 O mass fraction (25%~28%) to pH 6~8 and stir evenly.
[0046] (4) The solution obtained in step (3) was heated to 60° C. in a constant temperature water bath and kept for 2 hours to obtain a transparent sol.
Embodiment 2
[0048] The preparation of the palladium sol takes palladium acetate and citric acid as raw materials, and glacial acetic acid and distilled water as solvents.
[0049] The preparation steps are as follows:
[0050] (1) Weigh 120.8mg of Pd(CH 3 COO) 2 ﹒ 2H 2 O was dissolved in 15ml of glacial acetic acid and heated to obtain cloudy liquid A.
[0051] (2) Weigh 170.3 mg of citric acid and dissolve it in 10 ml of water to obtain solution B.
[0052] (3) Mix the turbid solution A prepared in step (1) with the solution B prepared in step (2), and add concentrated NH 3 ﹒ 2H 2 O (mass fraction 25% to 28%) until the pH is 6 to 8 and stirred evenly.
[0053] (4) The solution obtained in step (3) was heated to 70° C. in a constant temperature water bath and kept for 2 hours to obtain a transparent sol.
Embodiment 3
[0055] The preparation of palladium sol takes palladium chloride and citric acid as raw materials, and n-butanol, concentrated hydrochloric acid and distilled water as solvents.
[0056] The preparation steps are as follows:
[0057] (1) Weigh 159.8mg of PdCl 2 Dissolve in 15ml of n-butanol, add 10ml of concentrated hydrochloric acid dropwise and heat to obtain solution A.
[0058] (2) Weigh 500.4 mg of citric acid and dissolve it in 10 ml of n-butanol to obtain solution B.
[0059] (3) Mix solution A prepared in step (1) with solution B prepared in step (2), add concentrated NH 3 ﹒ 2H 2 O (mass fraction 25% to 28%) until the pH is 6 to 8 and stirred evenly.
[0060] (4) The solution obtained in step (3) was heated to 60° C. in a constant temperature water bath and kept for 2 hours to obtain a transparent sol.
[0061] (5) Dry the sol obtained in step (4) at 100° C. for several hours to form a wet gel, and heat-treat at 400° C. for 1 hour to obtain dry rubber powder.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com