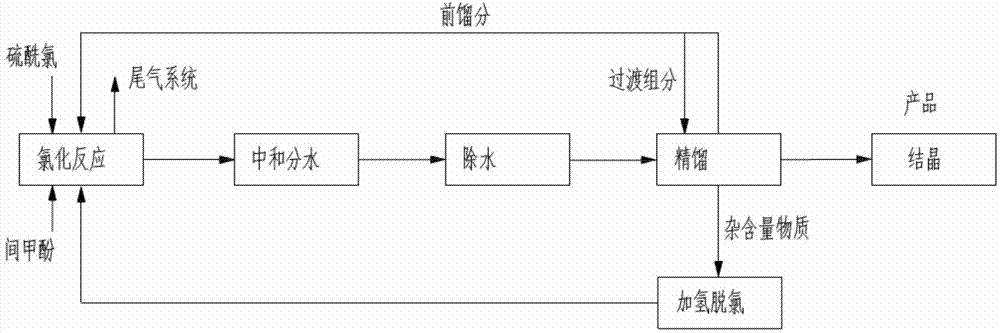
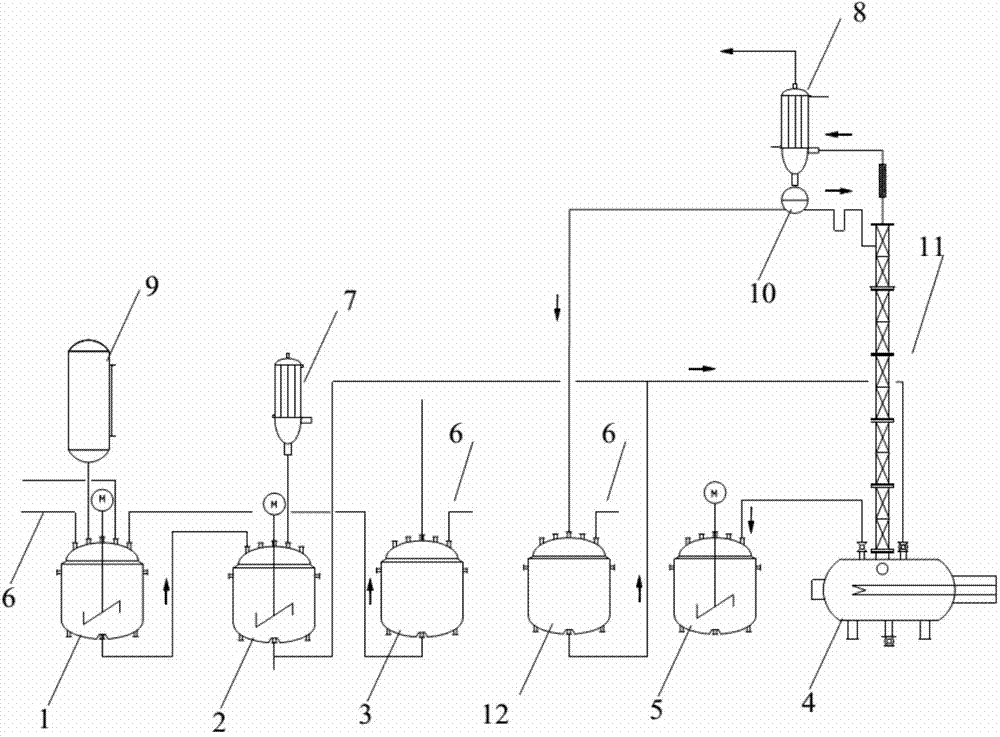
Synthesis method of 4-chloro-3-cresol and system thereof
A synthetic method, cresol technology, applied in the field of synthesis of carbocyclic compounds, can solve the problems of high theoretical plate number, increased production cost, environmental pollution, etc., and achieve the effect of improving the final yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 1mol (108g) of m-cresol to a 500g three-necked flask, under the condition of stirring at 300rpm, control the temperature of the three-necked flask at 30°C; add 0.9mol (124g) of sulfuryl chloride to the constant pressure funnel; add dropwise (2 hours) , the reaction temperature is controlled at 30°C, and the gas produced is treated with lye (Na at a concentration of 20wt%) 2 CO 3 )absorb. After the reaction, samples were taken for gas chromatography (GC) detection.
[0037] Table 1 embodiment 1 product test result
[0038] PCMC
MC
DCMC
OCMC
selectivity
yield
88.53
9.21
1.65
0.04
97.51%
88.53%
[0039] In the table, PCMC: 4-chloro-3-cresol, MC: m-cresol, DCMC: 4,6-dichloro-3-cresol (4,6-dichlorocresol), OCMC: 5-chloro- 3-cresol;
[0040] Yield=actual obtained quality / theoretical conversion should obtain quality*100%=conversion rate*selectivity*100%.
Embodiment 2
[0042] Add 1mol (108g) of m-cresol to a 500g three-neck flask, under the condition of stirring at 500rpm, control the temperature of the reaction kettle at 30°C; add 1.0mol (135g) of sulfuryl chloride into the constant pressure funnel; add dropwise (2.5 hours) , the reaction temperature is controlled at 30°C, and the gas generated is treated with lye (Na at a concentration of 25wt%) 2 CO 3 )absorb. After the reaction, samples were taken for GC detection, and the results are shown in Table 2.
[0043] Table 2 embodiment 1 product detection result
[0044] PCMC
Embodiment 3
[0046] Add 1mol (108g) m-cresol to a 500g three-necked flask, and control the temperature of the reaction kettle at 40°C under the condition of stirring at 400rpm; add 0.9mol (124g) sulfuryl chloride to the constant pressure funnel; perform dropwise addition (2 hours) , the reaction temperature is controlled at 40°C, and the gas produced is treated with lye (Na at a concentration of 10wt%) 2 CO 3 )absorb. After the reaction, samples were taken for GC detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com