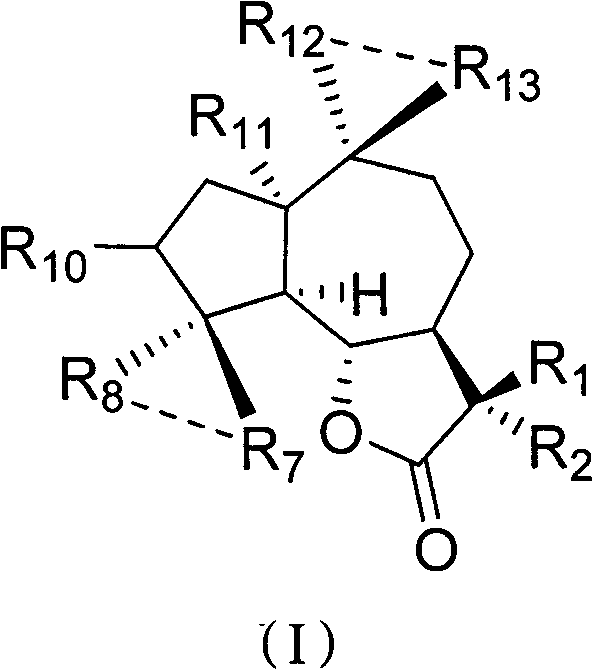
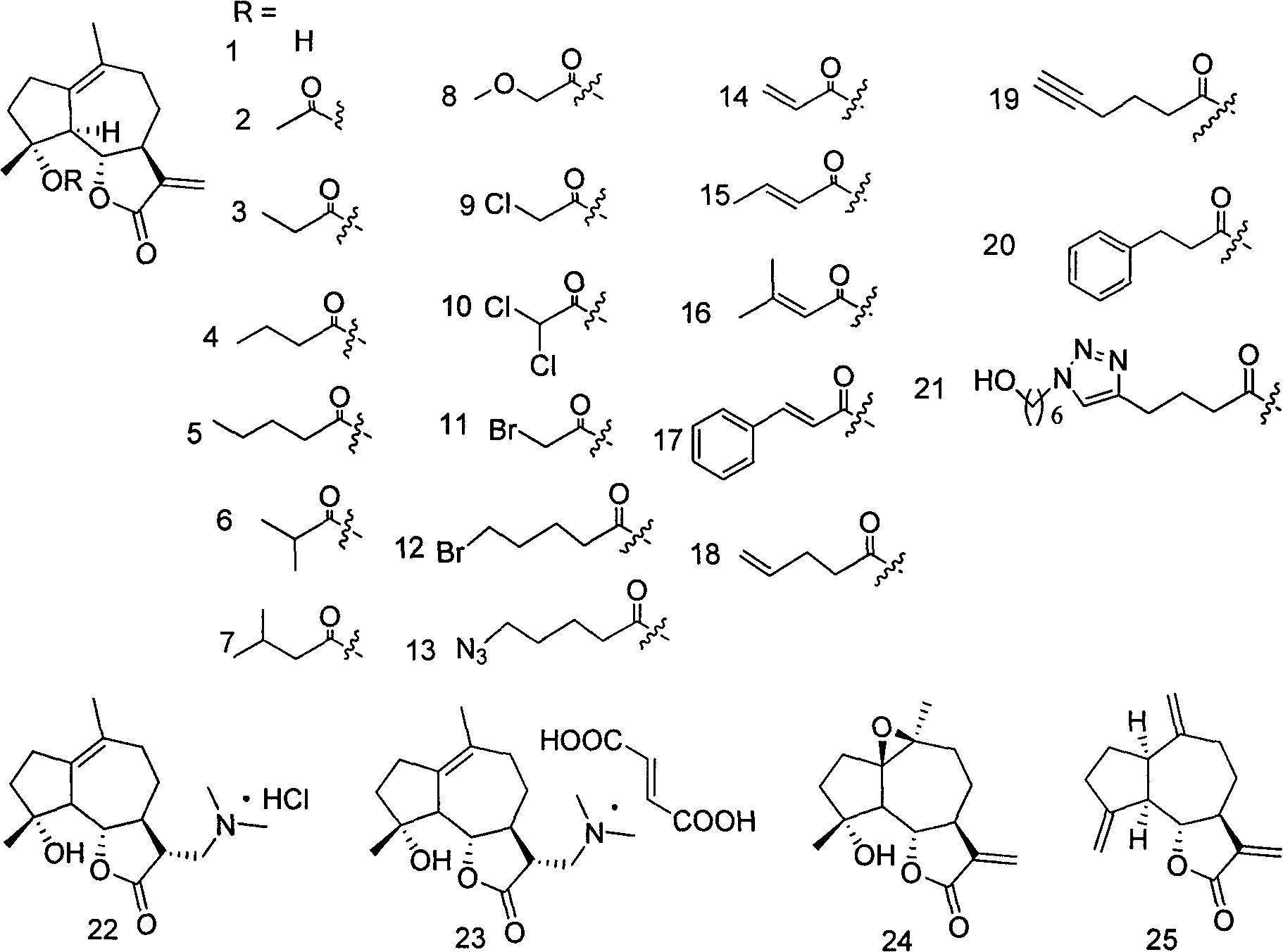
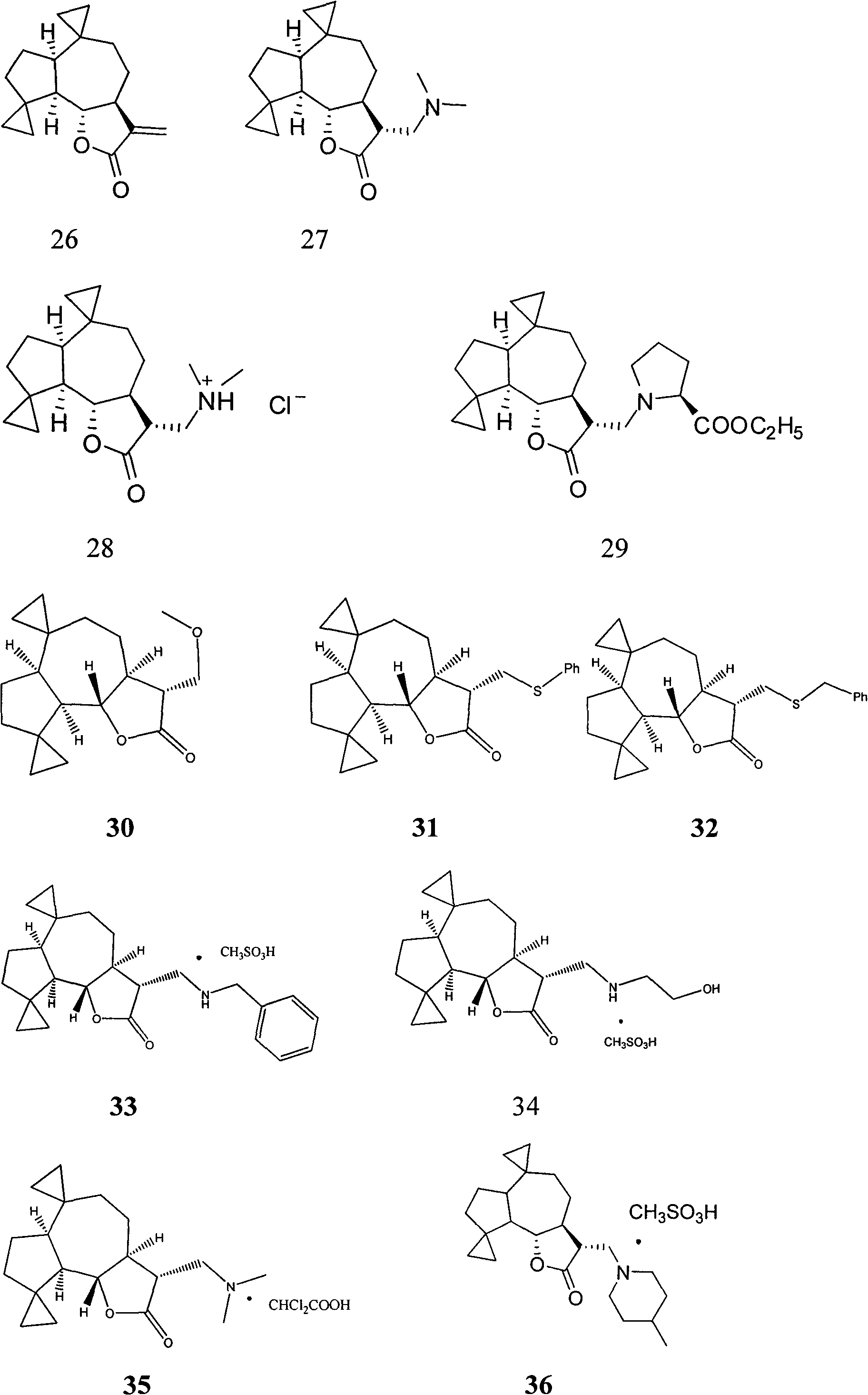
Applications of sesquiterpene lactone compound and derivative of sesquiterpene lactone compound in treatment of diabetes
A technology for diabetes and compounds, which is used in medical preparations containing active ingredients, metabolic diseases, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation method of compound 1-50
[0027] Preparation of Compound 1:
[0028] Parthenolide (50 mg, 0.2 mmol) was dissolved in 2.5 mL of dichloromethane, and p-toluenesulfonic acid (5 mg, 0.026 mmol) was added. The reaction system was left at room temperature and stirred overnight. Transfer the reaction solution to NaHCO 3 (10mL) in a saturated solution, collect the organic phase, extract the aqueous phase with a small amount of dichloromethane, combine the organic phases, and wash with Na 2 SO 4 After drying and filtering, the organic solvent was distilled under reduced pressure with a rotary evaporator, and purified on a silica gel column to obtain compound 1 (45 mg, yield 90%). 1 H NMR (CDCl3 , 400MHz) δ6.20(d, J=3.2Hz, 1H) 5.49(d, J=3.2Hz, 1H) 3.81(t, J=10.4Hz, 1H), 2.70(d, J=10.4Hz, 1H) , 2.65-2.62(m, 2H), 2.40-2.34(m, 1H), 2.07-2.26(m, 4H), 1.73-1.86(m, 2H), 1.68(s, 3H), 1.36-1.28(m, 4H); 13 C NMR (CDCl 3 , 100MHz) δ169.8, 138.7, 131.7,...
Embodiment 2
[0158] Example 2: Anti-diabetes and diabetes chronic complications activity test of compound 1-50
[0159] Chronic complications of diabetes are the main cause of disability and death in DM. Its pathogenesis involves polyol bypass, protein kinase C, hexosamine activation, and production of advanced glycation end products. In recent years, it has been found that hyperglycemia-induced mitochondrial response Increased production of sexual oxidation products is a possible common basis. Activation of reduced nicotinamide adenine dinucleotide oxidase is increasingly recognized as the central link in high glucose-induced oxidative stress. High glucose leads to protein uncoupling by causing Overexpression of mitochondrial peroxides causes oxidative stress and ultimately DNA damage. At the same time, from in vitro model tests and pathological examinations to epidemiological studies, many evidences have shown that inflammation is also one of the main pathogenesis of chronic complication...
Embodiment 3
[0172] Embodiment 3: injection
[0173] Compound 1-50 prepared in Example 1 was dissolved in a small amount of DMSO, added water for injection as usual, finely filtered, potted and sterilized to make an injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com