S-triazine derivatives and application thereof to organic electroluminescence devices
A technology of s-triazine and derivatives, applied in the field of s-triazine derivatives, can solve the problems of reducing luminous efficiency, limiting applications, reducing the efficiency of OLEDs, etc., and achieving the effect of high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
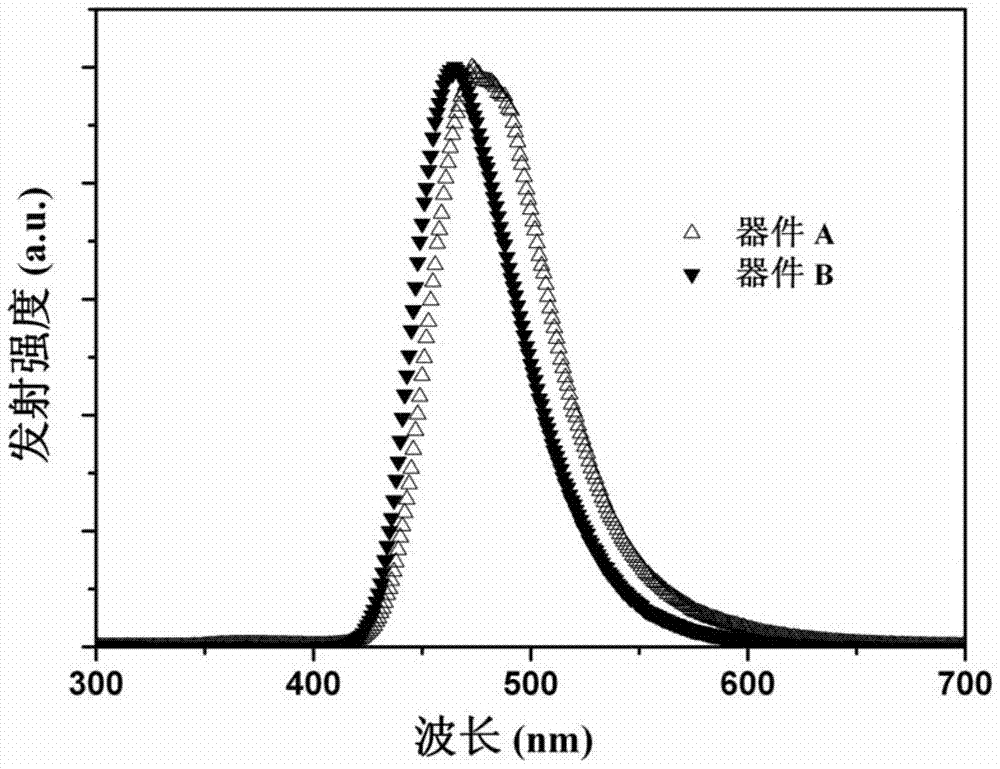
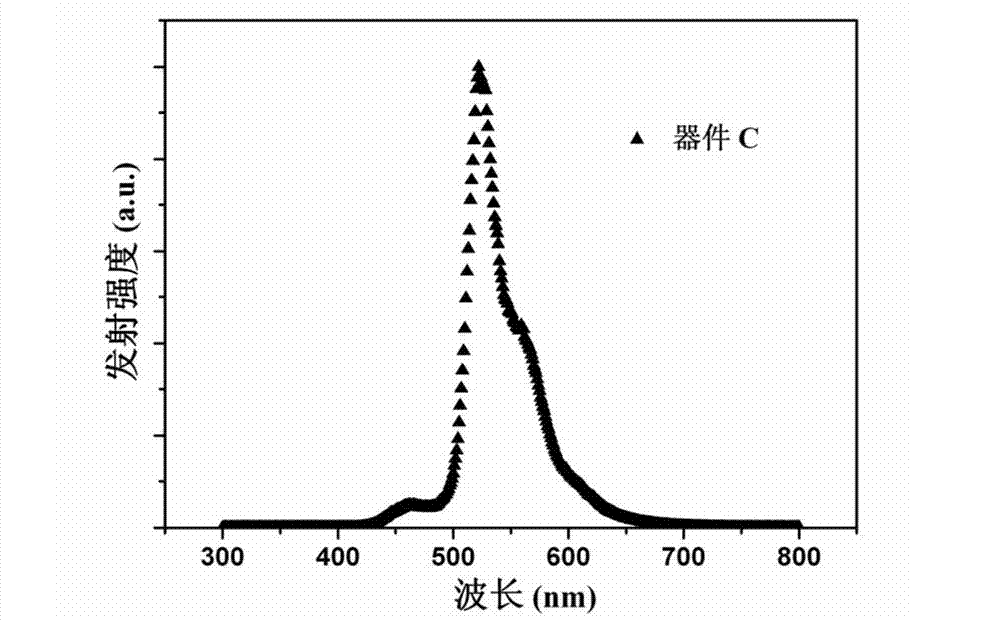
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1 compound TAr1 series compound
[0025] Synthesis of compound TAr1-1
[0026]
[0027] Under nitrogen protection, magnesium chips (1.152g, 48mmol) and anhydrous tetrahydrofuran (30mL) were placed in a three-necked flask, and a solution of bromobenzene (6.24g, 40mmol) in tetrahydrofuran (50mL) was added dropwise at reflux temperature. Reflux for 3 hours and cool to room temperature. Under nitrogen protection, the Grignard reagent was dropped into a solution of 1,3,5-trichloro-s-triazine (3.68g, 20mmol) in anhydrous tetrahydrofuran (40mL) at zero temperature, and the reaction was carried out at 20°C for 12 hours. After cooling to room temperature, the reaction was quenched with ammonium chloride solution. Extracted three times with dichloromethane, combined the organic phases, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, filtered and concentrated to obtain the crude product, and separated by col...
Embodiment 2
[0077] The synthesis of embodiment 2 compound TAr2 series compounds
[0078] Synthesis of compound TAr2-1
[0079]
[0080] Under nitrogen protection, magnesium chips (1.152g, 48mmol) and anhydrous tetrahydrofuran (30mL) were placed in a three-necked flask, and a solution of bromobenzene (6.24g, 40mmol) in tetrahydrofuran (50mL) was added dropwise at reflux temperature. Reflux for 3 hours and cool to room temperature. Under the protection of nitrogen, the Grignard reagent was dropped into a solution of 1,3,5-trichloro-s-triazine (2.44 g, 13.33 mmol) in anhydrous tetrahydrofuran (40 mL), and reacted at 40° C. for 12 hours after the dropping was completed. After cooling to room temperature, the reaction was quenched with ammonium chloride solution. The organic solvent was removed by rotary evaporation, dichloromethane (100mL) was added, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, filtered and concentrated to obtain the crude prod...
Embodiment 3
[0130] The synthesis of embodiment 3TAr3 series compound
[0131] Synthesis of compound TAr3-1
[0132]
[0133] Under nitrogen protection, magnesium chips (1.152g, 48mmol) and anhydrous tetrahydrofuran (30mL) were placed in a three-necked flask, and a solution of p-bromotoluene (6.8g, 40mmol) in tetrahydrofuran (50mL) was added dropwise at reflux temperature. , refluxed for 3 hours and then cooled to room temperature. Under the protection of nitrogen, the Grignard reagent was dropped into a solution of 1,3,5-trichloro-s-triazine (1.83 g, 10 mmol) in anhydrous tetrahydrofuran (30 mL). After the dropping was completed, the reaction was refluxed for 12 hours. After cooling to room temperature, the reaction was quenched with ammonium chloride solution. The organic solvent was removed by rotary evaporation, dichloromethane (100 mL) was added, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, filtered and concentrated to obtain a crude pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com