A method for determining the adsorption stability parameters of metal ions on a fixed metal affinity column
A technology of metal ions and stability constants, applied in the measurement of color/spectral characteristics, etc., can solve the problems of large error in measurement results, cumbersome detection process, poor reproducibility, etc., and achieve the effect of data stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] ( ) Preparation of epoxy silica gel: Weigh 2g of dry acidified silica gel, put it in a flask, add 40mL0.1mol / LNaAc-HAc buffer solution (pH4.0), sonicate for 2min, add 2mLγ-GLDP dropwise, and stir at 90°C The reaction was continued for 2 hours, cooled, filtered and washed with water until neutral, then dried and stored.
[0033] ( ) Preparation of aminocarboxylic acid chelating agent: take 30mL1mol / LNa 2 CO 3 , add 1.5g of ammonia carboxyl agent, adjust the pH to 8.5 after dissolving, and add 2g of epoxy silica gel to it. Stir and react continuously at 60-65°C for 12 hours, filter and wash with water, 10% HAc and water until neutral to obtain aminocarboxylic acid chelating agent.
[0034] ( ) Filling of aminocarboxylic acid chelating agent: Take 2g of aminocarboxylic acid chelating agent synthesized above, mix it with 40mL isopropanol as solvent, pour it into the packing column, and automatically fill it under 400kg pressure for 20min (10min isopropanol , 10min ...
Embodiment 1
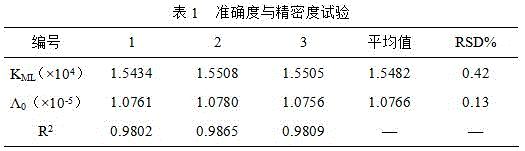
[0053] Embodiment 1 Accuracy and precision test
[0054] Preparation of L-Glu affinity column:
[0055] ( ) Preparation of epoxy silica gel: Weigh 2g of dry acidified silica gel, put it in a flask, add 40mL0.1mol / LNaAc-HAc buffer solution (pH4.0), sonicate for 2min, add 2mLγ-GLDP dropwise, and stir at 90°C The reaction was continued for 2 hr, cooled, filtered and washed with water until neutral and then dried and stored;
[0056] ( ) Preparation of L-Glu chelating agent: take 30mL1mol / LNa 2 CO 3 , add 1.5g L-Glu, adjust the pH to 8.5 after dissolving, and add 2g epoxy silica gel to it. Stir and react continuously at 60-65°C for 12 hours, filter and wash with water, 10% HAc and water until neutral to obtain L-Glu chelating agent;
[0057] ( ) Filling of L-Glu chelating agent: Take 2g of Glu-silica gel filler synthesized above, mix it with 40mL isopropanol as solvent, pour it into the packing column, and automatically fill it under 400kg pressure for 20min (10min isopr...
Embodiment 2
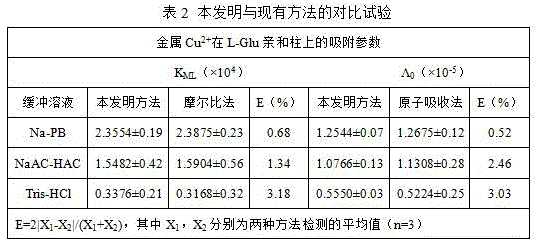
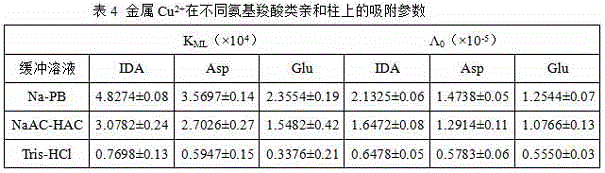
[0065] The method of the present invention is used for the determination of the bonding parameters of different metal ions on the L-glutamic acid (L-Glu) chromatographic column
[0066] Connect the L-Glu chromatographic column to the chromatographic system, and use 5mmol / L NaNO 2 -20mmol / L NaAC-HAC (pH5.0), 5mmol / L NaNO 2 -10mmol / L Na-PB (pH5.0), 5mmol / L NaNO 2 -15mmol / LTris-HCl (pH3.0) buffer solution, passed through the chromatographic column at a flow rate of 0.5mL / min at a wavelength of 356nm until the outflow curve reached the maximum absorption plateau, and blank breakthrough curves under different buffer systems were obtained. Respectively at 800nm, 510nm, 396nm wavelength at a flow rate of 0.5mL / min, in 20mmol / LNaAC-HAC (pH5.0), 10mmol / LNa-PB (pH5.0), 15mmol / LTris-HCl (pH3.0) 0.3mmol / L, 0.35mmol / L, 0.4mmol / L, 0.45mmol / L, 0.5mmol / LCuSO 4 、NiSO 4 、CoCl 2 Breakthrough curve of solution on L-Glu column. After each breakthrough curve of a metal ion is measured, the me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com