Method for preparing antigen to obtain antihydrophobic peptide antibody
一种疏水性、抗原的技术,应用在抗受体/细胞表面抗原/细胞表面决定因子免疫球蛋白、化学仪器和方法、抗动物/人类的免疫球蛋白等方向,能够解决小分子量等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0233] Antibody acquisition using antigen of the present invention (1), polyclonal antibody
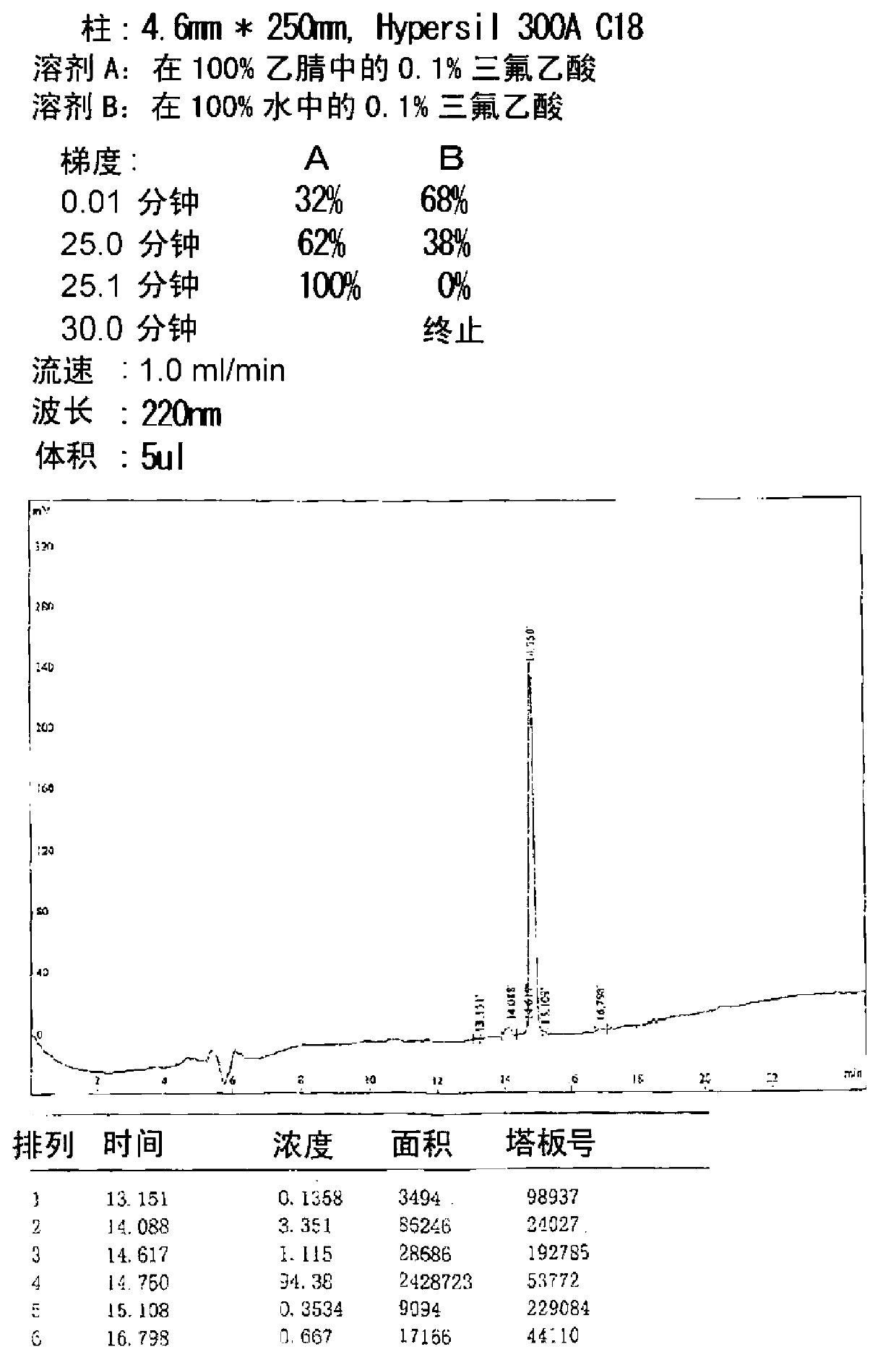
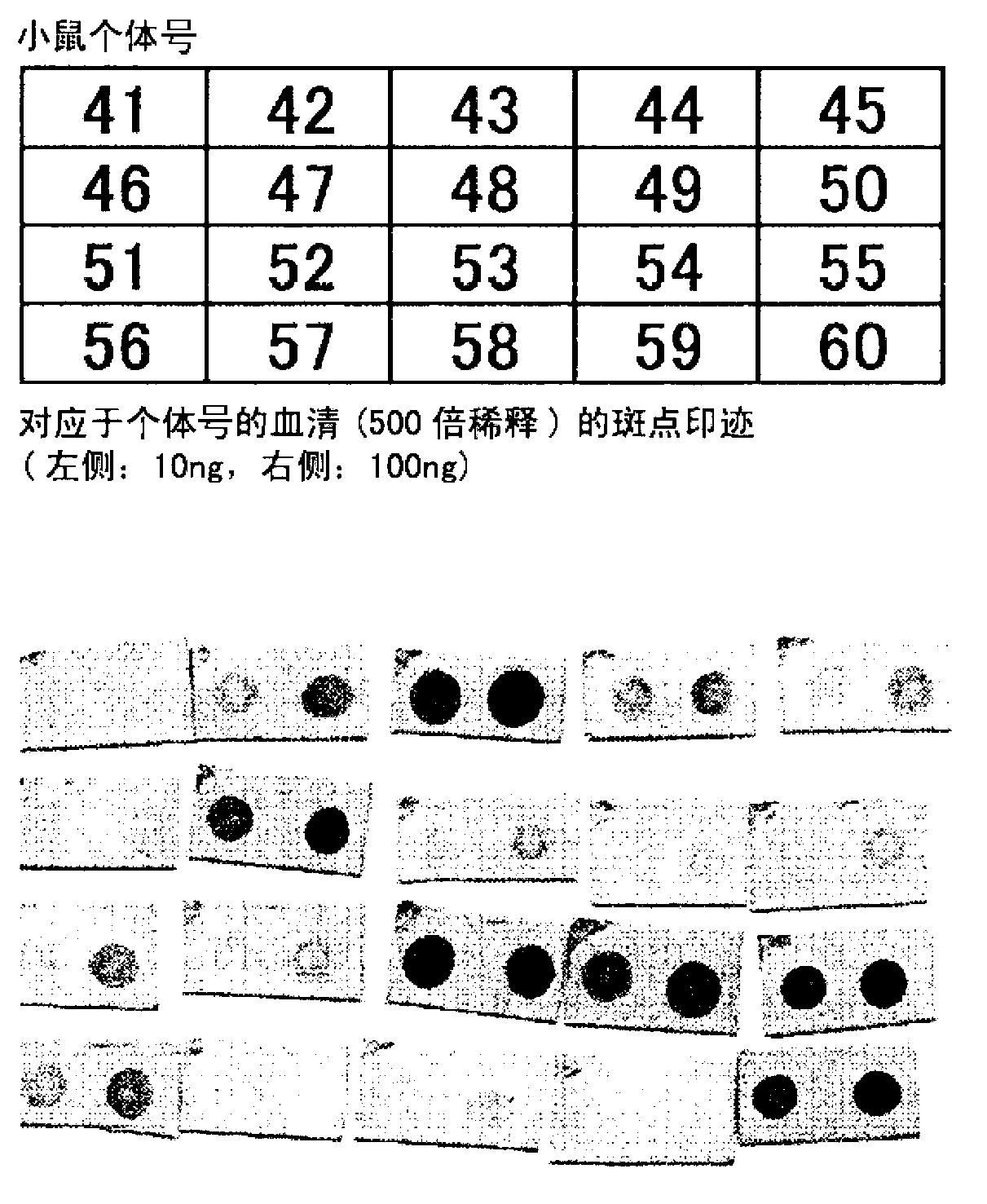
[0234] Titers were measured by dot blot using serum obtained by direct immunization using a solution of SPAPP suspended in a solvent containing a nonionic surfactant as an immunogen.
[0235] (1) Antigen preparation - SPAPP / 1% Tween 20
[0236] Six (6) mg of SPAPP with a purity of 92% was weighed and suspended in 5.4 ml of pure water. At this point, SPAPP was completely insoluble in aqueous solution and became cloudy. The solution was left to stand in this state for about 3 hours, and 0.6 ml of 10% Tween 20 was added and mixed. The final concentration of SPAPP was 2 mg / ml. Ultrasonic waves were applied intermittently for a total of about 3 minutes by a TOMY SEIKO Handy Sonic Model UR-20P at power level 7. Ultrasonic vibrations were applied until the transparency increased to some extent and the absorbance at 600 nm reached about 0.2. The resulting SPAPP solution was aliquoted into ...
Embodiment 2
[0244] Particle size distribution and molecular weight distribution of SPAPP
[0245] (1) Measurement of particle size distribution
[0246] The particle size distribution of the SPAPP / 1% Tween 20 suspension used in the above immunization method was measured as follows. After mixing the SPAPP suspension (2 mg / ml) and an equal amount of 0.4% trypan blue solution, the solution was applied to a cell counting device Countess (registered trademark) (manufactured by INVITROGEN). Particles from 2 μm to 80 μm can be measured in this device.
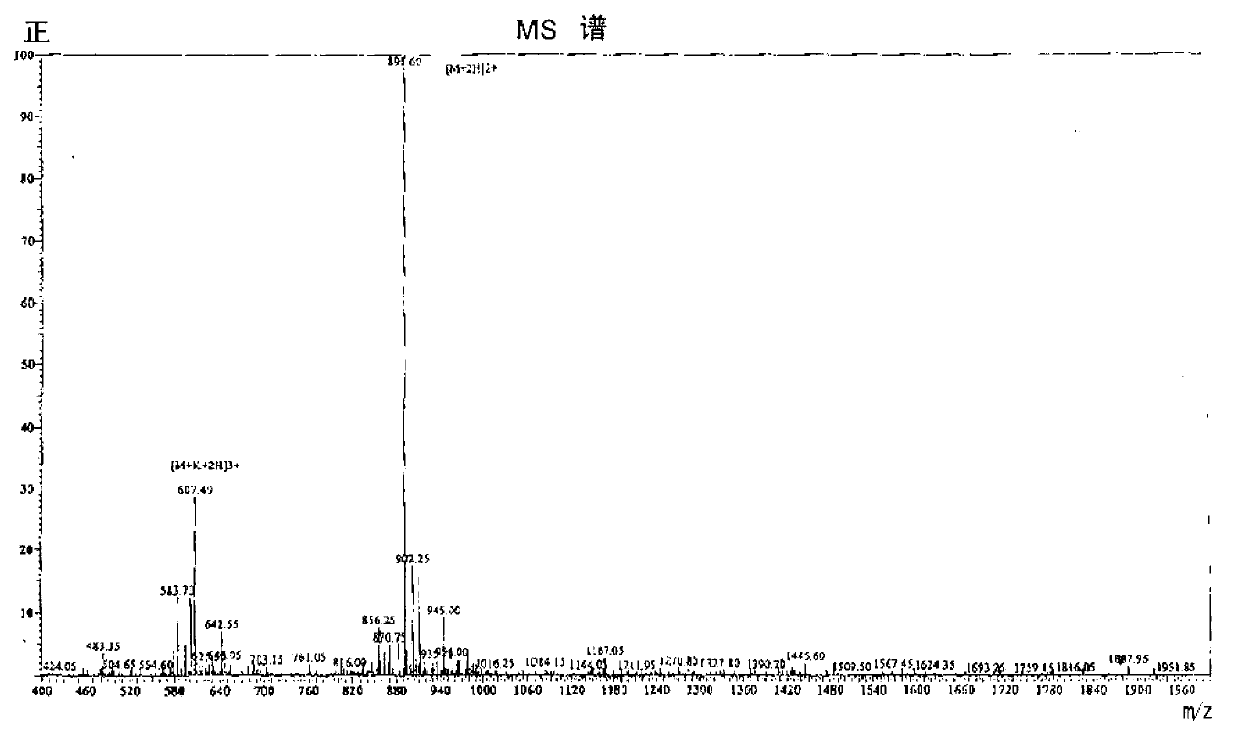
[0247] (2) Therefore, the particle size distribution of SPAPP in the suspension is 2 to 60 μm, and the concentration is 3.2×10 6 / ml. Most have dimensions of a few μm. Figure 4 The measurement results of the molecular weight distribution are shown.
[0248] The molecular weight distribution of SPAPP aggregates in suspension was measured according to the following procedure. Figure 5 An outline of the process is displayed.
[0249] (i) Th...
Embodiment 3
[0260] Aggregate Molecular Size (Molecular Weight / Particle Size) Distribution Test
[0261] (1) The relationship between the standing time of aggregates in pure water and the molecular size distribution
[0262] The time from suspending SPAPP in pure water to adding various surfactants was changed, thereby confirming that the molecular size distribution of aggregates changed according to time. For example, in the case of 1% Tween 20, the insoluble fraction (precipitation) was 5% when the surfactant was added quickly after suspending SPAPP in pure water, 17% when added after 1.5 hours, and 17% when the surfactant was added after 1.5 hours. 48% when added after 3 hours.
[0263] (2) Molecular size distribution in other nonionic surfactant solutions
[0264] Check whether SPAPP forms high molecular weight aggregates in other nonionic surfactant solutions. PEG60 Lauryl Ether (Polypure), Triton X-100 and Nonil P-40 were used as surfactants at 1% each. As in the case of the meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com