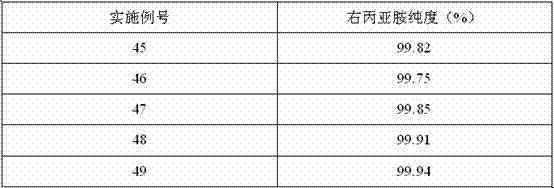
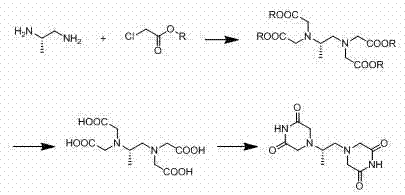
Preparation method of dexrazoxane
A technology of dextropropylimine and diaminopropane, which is applied in the field of preparation of dextropropylimine, can solve the problems that the product purity cannot be improved, the synthesis route of dextropropylimine is complicated, and the post-processing steps are cumbersome, etc. The effect of industrialized production, shortened production cycle, and increased environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of (S)-1,2-diaminopropane-tetraacetic acid methyl ester
[0044] Add 100 grams (0.68 moles) of (S)-1,2-diaminopropane hydrochloride, 324.76 grams (2.99 moles) of methyl chloroacetate, 283.3 grams (2.05 moles) of potassium carbonate, and 5 liters of acetone into 10 liters of reaction In the bottle, the temperature was raised to reflux for 16 hours. After the reaction was completed, the inorganic salt was filtered off, and the filtrate was concentrated to dryness to obtain a light yellow oil, which was dissolved in 2 liters of ethyl acetate and washed once with 0.2 liters of saturated sodium chloride solution. Dry over sodium sulfate, filter, and concentrate to obtain 195 g of methyl tetraacetate (yield of crude product: 79.2%).
[0045]
Embodiment 2
[0046] Embodiment 2: the preparation of (S)-1,2-diaminopropane-tetraacetic acid methyl ester
[0047] Add 50.4 grams (0.68 moles) of (S)-1,2-diaminopropane, 324.76 grams (2.99 moles) of methyl chloroacetate, 442 grams (3.20 moles) of potassium carbonate, and 5 liters of acetone into a 10-liter reaction flask, Heat up and reflux for 16 hours, the reaction is over, filter out the inorganic salts, concentrate the filtrate to dryness to obtain a light yellow oil, dissolve it in 2 liters of ethyl acetate, wash once with 0.2 liters of saturated sodium chloride solution, and wash with 50 grams of anhydrous sodium sulfate Dried, filtered, concentrated and dried to obtain 189 g of methyl tetraacetate (yield of crude product 76.8%).
[0048]
Embodiment 3
[0049] Embodiment 3: the preparation of (S)-1,2-diaminopropane-tetraacetic acid methyl ester
[0050] Add 100 grams (0.68 moles) of (S)-1,2-diaminopropane hydrochloride, 324.76 grams (2.99 moles) of methyl chloroacetate, 567 grams (4.1 moles) of potassium carbonate, and 5 liters of acetone into 10 liters of reaction In the bottle, the temperature was raised to reflux for 16 hours. After the reaction was completed, the inorganic salts were filtered off, and the filtrate was concentrated to dryness to obtain a light yellow oil, which was dissolved in 2 liters of ethyl acetate and washed once with 0.2 liters of saturated sodium chloride solution. Dry over sodium sulfate, filter, and concentrate to obtain 220 g of methyl tetraacetate (yield of crude product: 89.4%).
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com