anti-lrp6 antibody
A technology of antibodies and human antibodies, applied in the direction of antibodies, antibody medical components, antibody mimics/scaffolds, etc., can solve the problem that the role of LRP5/6 or FZD homodimerization has not been clearly defined
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0329] Experimental protocol
[0330] Cell Culture and Cell Assays
[0331]Cell lines EKVX and M14 were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum and 2 mM glutamine; JHH-1 cells were cultured in Williams' medium E with the same supplements. All other cell lines were obtained from the American Type Culture Collection (ATCC) and maintained as recommended.
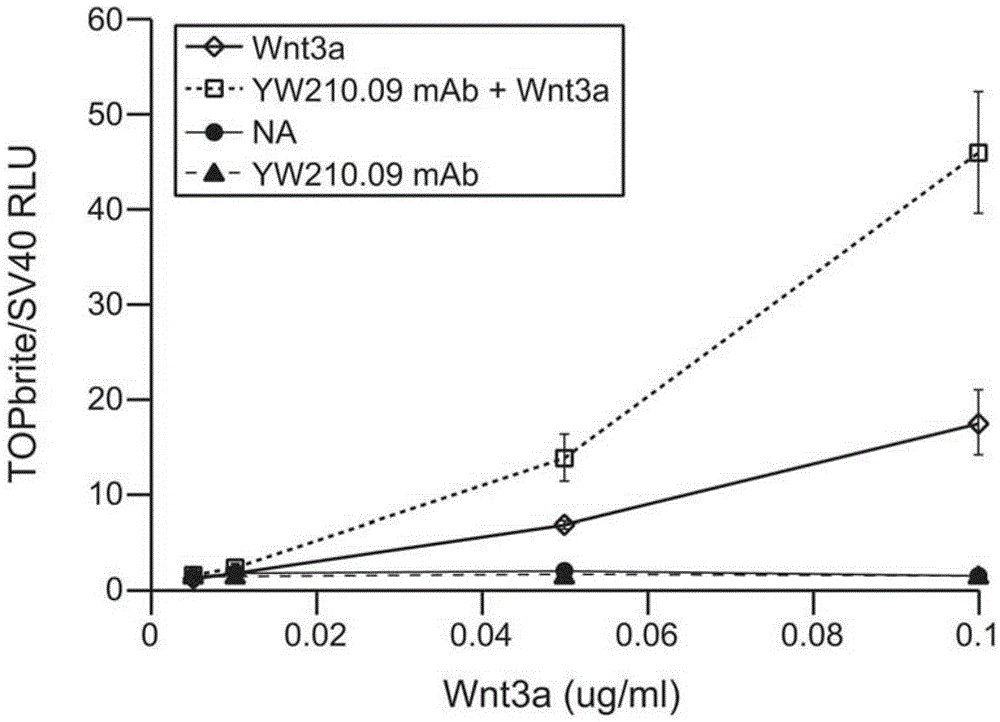
[0332] Cells were transfected with FuGENE6 transfection reagent (Roche) in 24-well plates according to the manufacturer's recommendations. For the luciferase reporter assay, a mix of transfected expression plasmid DNA: 7.5 ng TOPglow (Upstate) or TOPbrite (Zhang et al., 2009) firefly luciferase Wnt reporter, 0.5 ng pRL-SV40 Renilla luciferase (Promega ), and 1ngLEF1. Cells were treated with antibodies for 16-20 h, starting 24 h after transfection. Wnt3a protein (purified according to X, or purchased from R&D Systems) was added to the cells, starting 1 h after initiation of antibody treatment. ...
Embodiment 2
[0351] Isolation of Wnt-antagonistic and potentiating LRP6 monoclonal antibodies
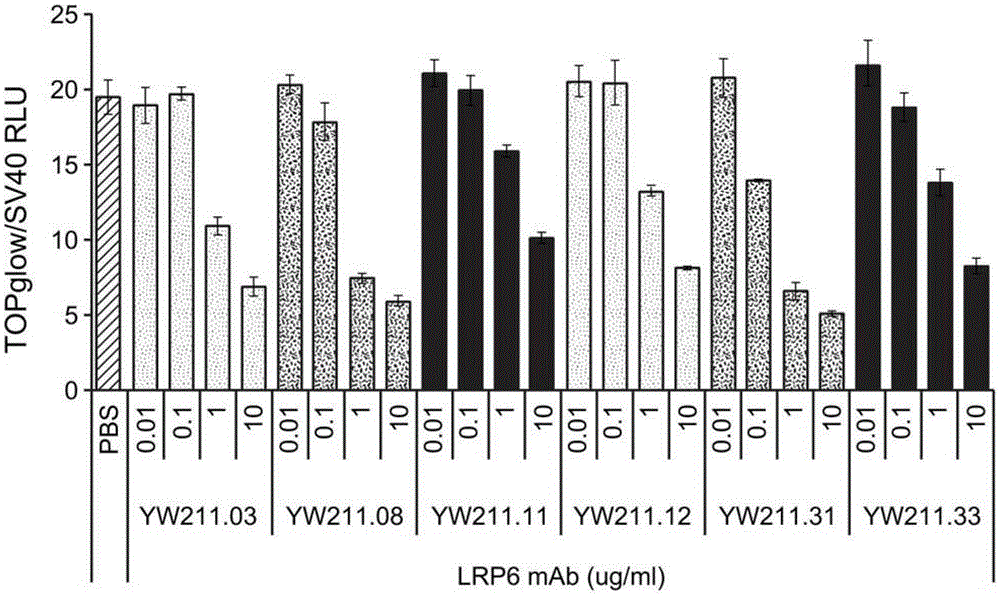
[0352] To develop candidate therapeutic molecules to manipulate Wnt signaling, antibodies capable of inhibiting or enhancing signaling induced by Wnt3a protein were generated. Recombinant LRP6.E1-E2-Fc (SEQ ID NO: 30) and LRP6.E3-E4-Fc (SEQ ID NO: 31 ) proteins were used to screen a human synthetic Fab phage display library and to confirm binding of LRP6 from isolated phage clones by ELISA. Twenty-four unique antibody heavy chain clones against LRP6.E1-E2 and 22 clones against LRP6.E3-E4 were isolated, reformatted and expressed human IgG1 antibodies. Six LRP6.E3-E4 antibodies inhibited Wnt luciferase reporter activity in HEK293 cells induced with 0.1 mg / ml purified Wnt3a in a concentration-dependent manner ( Figure 1A . Error bars for this and all other graphs represent the standard deviation of at least 3 replicates unless otherwise indicated). These antibodies were named YW211.03, YW211.0...
Embodiment 3
[0356] Effect of LRP6 monoclonal antibody on autocrine Wnt signaling
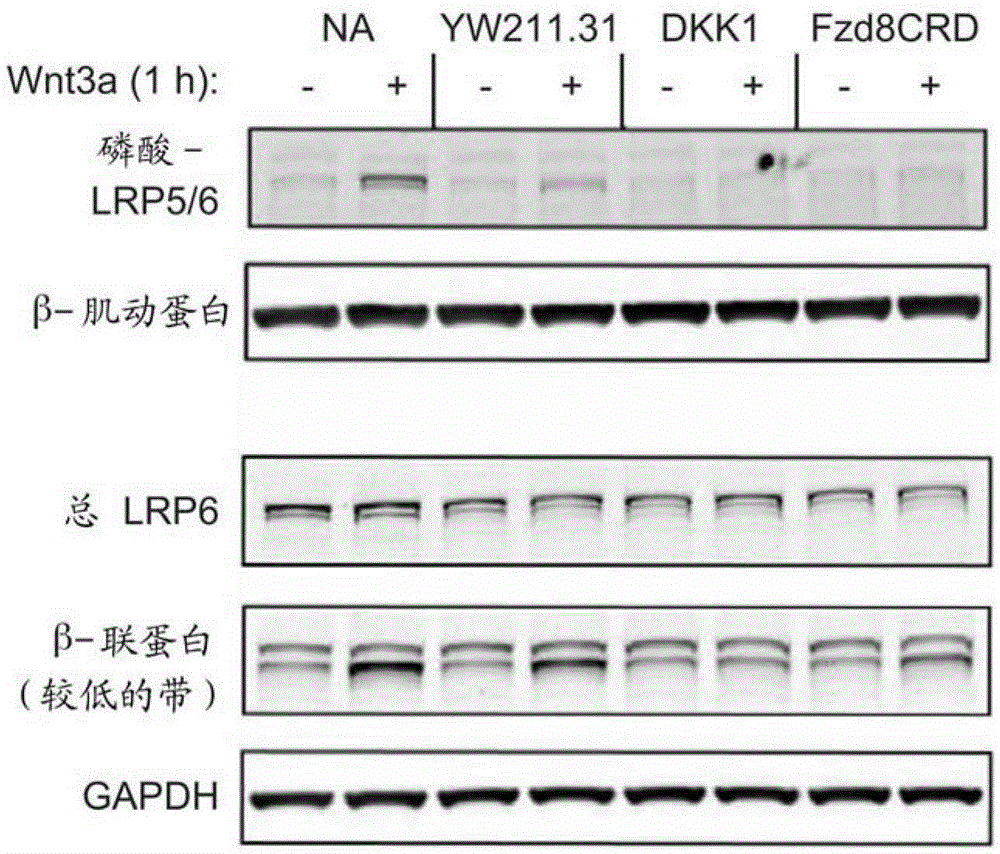
[0357] The ability of LRP6 antibodies to antagonize or potentiate endogenous or autocrine Wnt signaling was determined using various tumor cell lines (Bafico et al., 2004; DeAlmeida et al., 2007; Akiri et al., 2009). In teratoma cell lines PA-1 and NTERA-2, the YW211.31 antibody inhibited reporter activity induced by autocrine Wnt signaling with potency similar to that observed for exogenous Wnt3a ( Figure 2A , showing concentration-dependent inhibition of autocrine Wnt signaling in PA-1 teratoma carcinoma cells transfected with a luciferase reporter and treated with LRP6 antibody alone or in combination, or Fzd8CRD-Fc protein (positive control) and strengthen).
[0358] In PA1 cells, inhibition of Wnt signaling by the YW211.31 antibody was also observed for the expression of endogenous Wnt target genes ( Figure 2B ). Figure 2B Shown with or without 0.3mg / mlWnt3a protein treatment, and with 10mg / mlY...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com