Anti-fungal agent
An antifungal agent, frankincense technology, applied in antifungal agents, plant/algae/fungus/moss components, plant raw materials, etc., can solve problems such as medical field troubles, and achieve the effect of high antifungal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057]
[0058] After pulverizing mastic resin (20.5 g) in a mortar, the pulverized mastic resin was separated into n-hexane (400 mL) and methanol (400 mL).
[0059] Then, the n-hexane layer and the methanol layer were separated using a separatory funnel, and then the methanol layer was subjected to the same separation operation using n-hexane (300 mL) again three times.
[0060]Then, the n-hexane layer (about 900 mL) and the methanol layer (about 650 mL) were concentrated under reduced pressure in an evaporator at 40 degrees to obtain mastic n-hexane extract (11.5 g) and methanol extract (8.0 g).
[0061] Further, silica gel (about 70 g, silica gel 60, manufactured by Merck) column chromatography (inner diameter: 2.5 cm, height: 30 cm) was performed on the mastic n-hexane extract (1.02 g).
[0062] That is, as the column elution solvent, 1.1 L of n-hexane / ethyl acetate (10 / 1) was used, and then 0.8 L of n-hexane / ethyl acetate (7 / 1) was used.
[0063] Then, about 10 mL of t...
Embodiment 2
[0067]
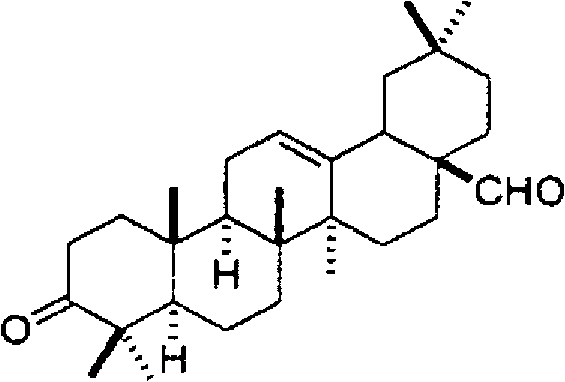
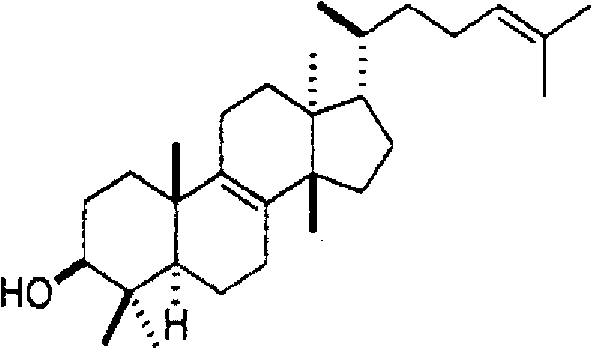
[0068] For the fractionated Fr.1, Fr.3, Fr.5-1, Fr.5-2, Fr.6 and Fr.8, a gas chromatography mass spectrometer (QP5050A manufactured by Shimadzu Corporation) and a nuclear magnetic resonance device ( Structural analysis was performed by NMR (nuclear magnetic resonance) (Unity 600 manufactured by Valian Corporation). The result is shown below.
[0069] [chemical formula 1]
[0070]
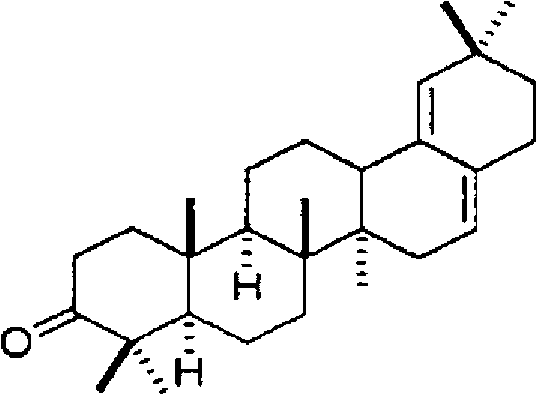
[0071] As a result of structural analysis of Fr. 1, it was found that it is 3-oxo-28-norolean-16,18-diene represented by the above chemical formula 1. In addition, the structural analysis data of Fr. 1 are shown below.
[0072] EIMS (relative intensity) (m / z): 408 (M + ), 393 (M-15), 207;
[0073] 1 H-NMR (CDCl 3 , 600MHz): 0.77 (3H, s), 0.95 (3H, s), 0.96 (3H, s), 0.97 (6H, s), 1.01 (6H, s), 1.07 (3H, s), 5.15 (1H, brs), 5.28 (1H, m);
[0074] 13 C-NMR (CDCl 3 , 150MHz): 14.5, 16.3, 16.6, 19.5, 21.0, 21.1, 25.1, 26.9, 27.9, 28.8, 29.8, 31.0, 32.6, 32.9, 33.8, 34.1, 36.8, 37....
Embodiment 3
[0105]
[0106] First, the effects of mastic n-hexane extract (hereinafter referred to as "MH") and methanol extract (hereinafter referred to as "MM") on drug-resistant bacteria were determined.
[0107] In addition, the bacterial strains used are the following four types that cause troubles in the medical field.
[0108] (1) Methicillin-resistant Staphylococcus aureus (MRSA)
[0109] (2) Vancomycin-resistant Enterococcus (VRE)
[0110] (3) Escherichia coli (E.coli)
[0111] (4) Pseudomonas aeruginosa (P.aeruginosa)
[0112] Dissolve MH and MM separately in acetone. The resulting MH solution was placed on paper disks for antibiotic testing (8 mm thick, ADVANTEC (registered trademark)) to prepare test disks infiltrated at 5 mg / disc and test disks infiltrated at 20 mg / disc.
[0113] Also, similarly, the obtained MM solution was placed on paper disks for antibiotic testing (8 mm thick, ADVANTEC (registered trademark)) to prepare test disks infiltrated at 5 mg / disc and test ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com