Fluoroquinolone acetal ftivazide as well as preparation method and application thereof
A fluoroquinolone aldehyde and quinolone aldehyde technology, which is applied in the field of fluoroquinolone derivative compounds, can solve the problems of poor tuberculosis efficacy, phototoxicity, and influence on animal cartilage development, etc., so as to reduce the probability of drug resistance, improve the inhibitory effect, and reduce toxicity. Effects of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
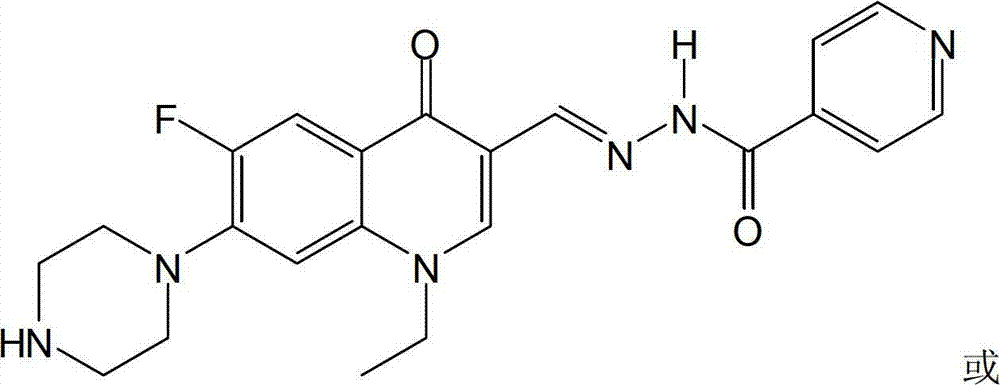
[0040] The fluoroquinolone aldehyde isoniazone of this embodiment is N′-[1-cyclopropyl-6-fluoro-7-(piperazin-1-yl)-quinoline(1H)-4-one-3-ylidene Methyl] isonicotinohydrazone, its chemical structure is:
[0041]
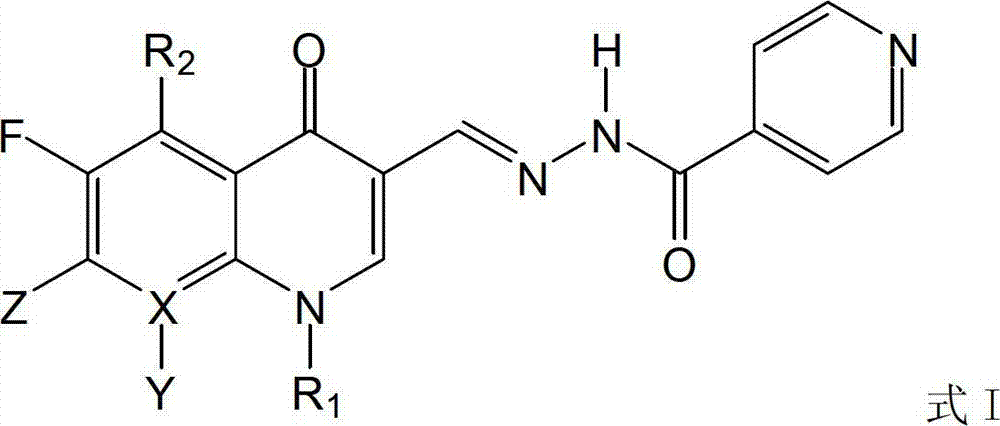
[0042] R in formula I 1 Is cyclopropyl, R 2 Is a hydrogen atom, X is a carbon atom, Y is a hydrogen atom, Z is a substituent 1, R 3 Is a hydrogen atom, R 4 Is a hydrogen atom.
[0043] The preparation method of the fluoroquinolone aldehyde isoniazone in this embodiment is: take 1-cyclopropyl-6-fluoro-7-(piperazin-1-yl)-3-formyl-4(1H)-quinoline 0.32g (1mmol) of ketone, 15ml of absolute ethanol, heat to dissolve, then add 0.14g (1mmol) of isoniazid and reflux for 5h. After standing overnight, the resulting solid was collected by filtration, washed with absolute ethanol, and dried to obtain 0.4 g of the fluoroquinolone isoniazone compound of Example 1, with a yield of 93%, m.p. 290-292°C. 1 HNMR(DMSO-d 6 , 400MHz) δ: 11.98 (s, 1H, CONH), 8.77 (s, 1H, C 2 -H), 8.75(d, J=8.7Hz...
Embodiment 2
[0045] The fluoroquinolone aldehyde isoniazone in this embodiment is N′-[1-ethyl6-fluoro-7-piperazin-1-yl-quinoline(1H)-4-one-3-methylene]iso Cigarette hydrazone, its chemical structure is:
[0046]
[0047] R in formula I 1 Is ethyl, R 2 Is a hydrogen atom, X is a carbon atom, Y is a hydrogen atom, Z is a substituent 1, R 3 Is a hydrogen atom, R 4 Is a hydrogen atom.
[0048] The preparation method of the fluoroquinolone aldehyde isoniazone in this embodiment is: take 1-ethyl-6-fluoro-7-(piperazin-1-yl)-3-formyl-4(1H)-quinolinone 0.31g (1mmol), 15ml of absolute ethanol, heat to dissolve, then add isoniazid 0.14g (1mmol), reflux for 4h. After standing overnight, the resulting solid was collected by filtration, washed with absolute ethanol, and dried to obtain 0.35 g of the fluoroquinolone isoniazone compound of Example 2 with a yield of 83%, m.p. 166-169°C. 1 HNMR(CD 3 CO 2 D, 400MHz) δ: 8.88~8.90 (m, 3H, C 2 -Handpyridine-H), 8.82(s, 1H, N=CH), 8.12(d, J=6.4Hz, 2H, pyridine-H), 8...
Embodiment 3
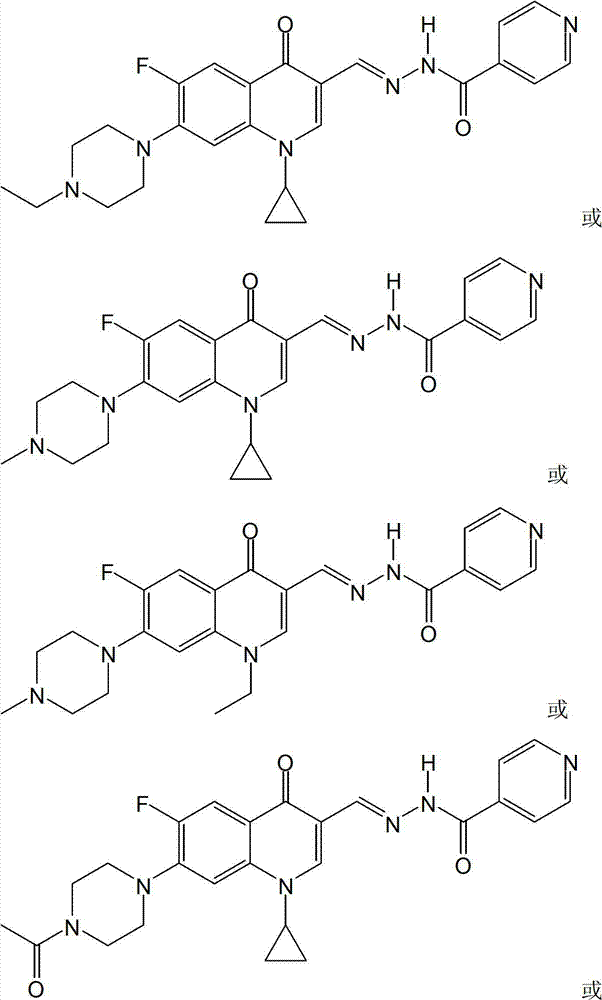
[0050] The fluoroquinolone aldehyde isoniazone in this embodiment is N′-[1-cyclopropyl-[6-fluoro-7-(4-ethylpiperazin-1-yl)-quinoline(1H)-4- Keto-3-methylene] isoniazone, its chemical structure is:
[0051]
[0052] R in formula I 1 Is cyclopropyl, R 2 Is a hydrogen atom, X is a carbon atom, Y is a hydrogen atom, Z is a substituent 1, R 3 Is ethyl, R 4 Is a hydrogen atom.
[0053] The preparation method of the fluoroquinolone aldehyde isoniazone in this embodiment is: take 1-cyclopropyl-6-fluoro-7-(4-ethylpiperazin-1-yl)-3-formyl-4(1H )-Quinolinone 0.34g (1mmol), 15ml of absolute ethanol, heated to dissolve, then add isoniazid 0.14g (1mmol), reflux for 4h. Placed overnight, the resulting solid was collected by filtration, washed with absolute ethanol, and dried to obtain 0.38 g of the fluoroquinolone isonicotinohydrazone compound of Example 3, with a yield of 83%, m.p. 281.8-283.6°C. 1 HNMR(CD 3 CO 2 D, 400MHz) δ: 8.83~8.86 (m, 3H, C 2 -Handpyridine-H), 8.75(s, 1H, N=CH), 8.06(d, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com